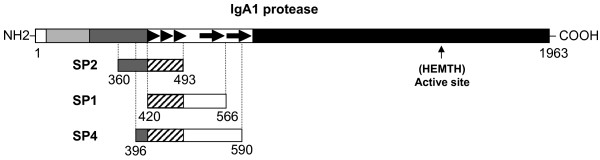
Figure 1.
Schematic representation of the selected phage clones. Alignment of the selected antigen fragments with the S. pneumoniae IgA1 protease of strain R6. The streptococcal IgA1 protease is typically organised in (i) a signal peptide (white box), (ii) a 200 amino acid N-terminal domain with three transmembrane segments and an LPXTG anchor domain (light grey box), (iii) a 200 amino acid fibrillar domain (grey box), (iv) a region with repeat segments possibly involved in the binding of extracellular matrix components (empty box with arrows), and (v) a large central and C-terminal domain containing the active site of the protease (black box). The repeat segments in R6 are three repeats of seventeen amino acids with a conservation of 62 to 75% followed by two repeats of 78 amino acids with 64% identity. The common region of the antigen fragments in shown in diagonal bars.