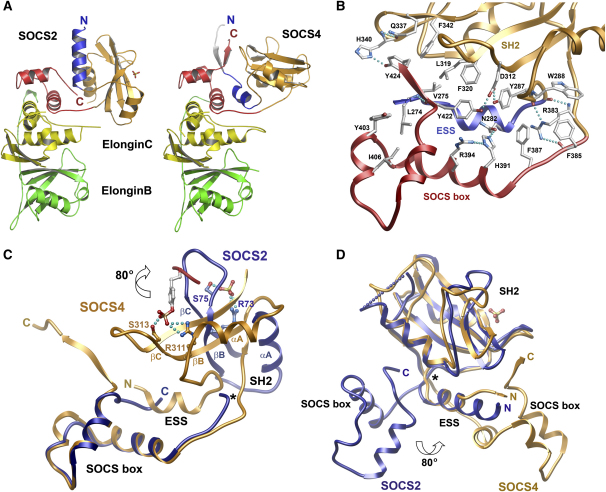
Figure 3.
Alternative Domain Organization in the SOCS2 and SOCS4 Ternary Complexes
(A) Comparison of the SOCS2-ElonginB/ElonginC and SOCS4-ElonginB/ElonginC structures highlighting the switch in packing between the SOCS2/SOCS4 C terminus and the N-terminal ESS helix (colored as in Figure 1).
(B) Molecular interactions stabilizing the domain organization in SOCS4.
(C) Structural overlay of the SOCS2 and SOCS4 SOCS box showing an 80° rotation of the SOCS4 SH2 domain relative to the SOCS2 SH2. The positions of the SOCS2 and SOCS4 phosphotyrosine pockets are indicated by a SOCS2-bound sulfate ion and a SOCS4-bound phosphotyrosine (modeled as in Figure 2). For clarity, the SOCS2 ESS is omitted and only the N-terminal half of each SH2 domain is shown. An asterisk denotes the position of the hinge point for rotation which occurs at R383/T384 in SOCS4.
(D) Structural overlay of the SOCS2 and SOCS4 SH2 domains showing the alternative packing sites for the respective SOCS box domains on opposite faces of the ESS and SH2. The SOCS2-bound sulfate ion indicates the position of the SH2 phosphotyrosine pocket.