Figure 2. Homology model of the haem-binding domain of the human soluble guanylate cyclase (sGC) β-subunit.
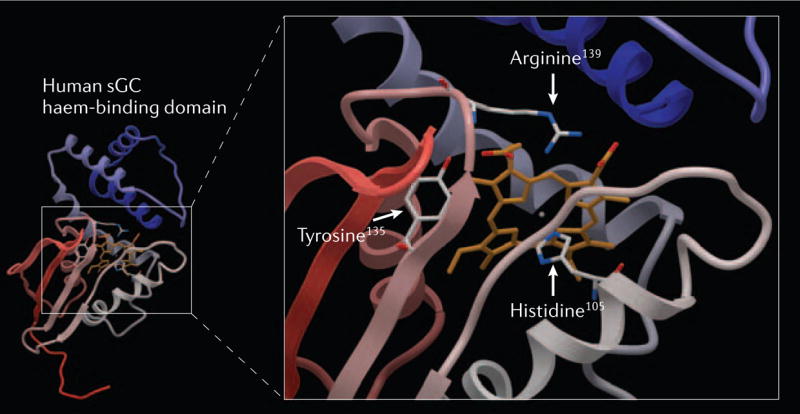
The model depicted is based on the recently resolved crystal structure of a prokaryotic haem-binding protein of Thermoanaerobacter tengcongensis with sequence homology to the sGC haem-binding domain16,17. Residues responsible for the coordination of the haem are shown in the enlargement on the right side. The axial haem ligand histidine-105 and the counterparts of the haem propionic acids tyrosine-135, serine-137 and arginine-139 comprise the unique sGC haem-binding motif Y-x-S-x-R (serine-137 was omitted for clarity)18,19,83.
