Abstract
Objective. Acute pancreatitis is one the important causes of systemic inflammatory response syndrome (SIRS). SIRS results in gut barrier dysfunction that allows bacterial translocation and pancreatic infection to occur. Indomethacin has been used to reduce inflammatory process and bacterial translocation in experimental models. The purpose of this study was to determine the effect of inhibition of prostaglandin E2 (PGE2) production on pancreatic infection. Materials and methods. An experimental model of severe acute pancreatitis (AP) was utilized. The animals were divided into three groups: sham (surgical procedure without AP induction); pancreatitis (AP induction); and indomethacin (AP induction plus administration of 3 mg/kg of indomethacin). Serum levels of interleukin (IL)-6 and IL-10, PGE2, and tumor necrosis factor (TNF)-α were measured 2 h after the induction of AP. We analyzed the occurrence of pancreatic infection with bacterial cultures performed 24 h after the induction of AP. The occurrence of pancreatic infection (considered positive when the CFU/g was >105), pancreatic histologic analysis, and mortality rate were studied. Results. In spite of the reduction of IL-6, IL-10, and PGE2 levels in the indomethacin group, TNF-α level, bacterial translocation, and pancreatic infection were not influenced by administration of indomethacin. The inhibition of PGE2 production did not reduce pancreatic infection, histologic score, or mortality rate. Conclusion. The inhibition of PGE2 production was not able to reduce the occurrence of pancreatic infection and does not have any beneficial effect in this experimental model. Further investigations will be necessary to discover a specific inhibitor that would make it possible to develop an anti-inflammatory therapy.
Keywords: experimental acute pancreatitis, pancreatic infection, indomethacin, bacterial translocation, prostaglandin E2
Introduction
It is known that acute severe pancreatitis (AP) is an abdominal catastrophe and has a high mortality rate 1. At the present time, no specific therapy has been shown to be uniformly effective in reducing morbidity and mortality 1,2.
Severe AP is one of the most important causes of systemic inflammatory response syndrome (SIRS) 3. After the initial injury to the pancreas acinar cell a local inflammation of the pancreas is responsible for triggering a sequence of events that ends in a generalized inflammatory response that results in multiple organ damage 4.
Knowledge about the pathophysiology of AP has increased considerably in the past few years. However, acute severe pancreatitis remains a harmful disease with a high mortality rate. The systemic inflammatory response is mediated by cytokines such as IL-1, IL-6, IL-8, TNF-α, prostaglandin E2 (PGE2), platelet-activating factor (PAF), and C-reactive protein 5. There is now evidence that plasma levels of TNF-α, IL-1, and IL-6 increase early in the course of AP and they are correlated with the severity of the disease. Furthermore, a high level of inflammatory mediators is responsible for fuelling the SIRS 6.
SIRS results in several organ dysfunctions such as respiratory distress, renal failure, homodynamic instability, and gut barrier dysfunction. The gut barrier dysfunction allows the occurrence of bacterial translocation. Mucosal barrier damage during AP leads to an increase in permeability, which promotes the escape of bacteria from the gut 7. Additionally, it has been speculated that release of excessive amounts of inflammatory mediators leads to failure of the intestinal barrier 8. Therefore, the occurrence of bacterial translocation in AP can be considered an indirect marker of systemic inflammatory response level.
Pancreatic infection is the most serious complication of severe AP, with a mortality rate up to 80%. The pathogenesis of pancreatic infection in AP remains poorly understood. Increased gut bacterial translocation in AP has been demonstrated in a number of previous studies, where bacterial translocation has been correlated with pancreatic infection 9,10. Our group previously demonstrated the occurrence of bacterial translocation in experimental AP 11,12.
PEG2 and prostaglandin-derived mediators play an important role in mediating the systemic inflammatory response. Cyclooxygenases (COX) are the key enzymes of prostaglandin synthesis. Inhibition of PEG2 production could reduce the systemic inflammatory response and therefore have some benefits in AP 13,14.
Indomethacin is an unselective COX inhibitor capable of reducing PEG2 production. In experimental models of shock and burn injury, administration of PEG2 synthesis blocker prevented the occurrence of bacterial translocation. It has been reported that high levels of PEG2 are responsible for suppression of splenic T-cell proliferation during sepsis resulting in bacterial translocation 15. However, the role of reduction of PEG2 production in cytokine production and pancreatic infection has not been evaluated in experimental AP.
To elucidate the role of PEG2 in severe AP we sought to analyze the effects of inhibition of PEG2 production on the local and systemic response in experimental severe AP.
Materials and methods
Animals
One hundred twenty male Wistar rats, weighing 225–250 g, were housed in individual cages. The temperature was kept at 20–22°C, a 12 h light-dark cycle was maintained, and all rats were fed with a standard rat chow and water ad libitum during the entire protocol; any fast period was observed. The experimental protocol was approved by the University of São Paulo Ethics Commission. All animals received care in accordance with ‘Guide for the Care and Use of Laboratory Animals.’
Experimental design
Animals were divided into three experimental groups: group I (sham group) – 40 rats submitted to surgical procedure without induction of AP plus intraperitoneal administration of 0.5 ml of saline; group II (pancreatitis group) – 40 rats submitted to induction of AP by sodium taurocholate injection into the pancreatic duct plus intraperitoneal administration of 0.5 ml of saline; and group III (indomethacin group) – 40 rats submitted to induction of AP by sodium taurocholate injection into the pancreatic duct plus intraperitoneal administration of 3 mg/kg of indomethacin.
Induction of acute pancreatitis
The rats were operated under aseptic conditions, using ketamine anesthesia 0.2 ml/100 g (Ketalar®). The pancreas was exteriorized through a midline abdominal incision, the proximal bile duct was clamped at the liver hilum level, and the distal bile duct was cannulated using a 19G polyethylene catheter through the duodenal wall. AP was induced by intraductal retrograde injection of 0.5 ml of 2.5% sodium taurocholate (Sigma-Chemical Company™, St Louis, MO, USA), with pressure control. Injection flow was 0.2 ml/min. In the sham group, sodium taurocholate injection was omitted, but the surgical procedure was identical to the other groups, including bile duct cannulation.
Administration of indomethacin and saline
Intraperitoneal administration of 3 mg/kg of indomethacin (Sigma-Chemical Company™) was performed after the induction of AP and the same dose was repeated each 12 h.
Intraperitoneal administration of 0.5 ml of saline was performed after the induction of AP and was repeated every 12 h in groups I and II.
The administration of indomethacin and saline was carried out in aseptic conditions.
Levels of cytokines
Blood samples were collected 2 h after the induction of AP by needle aspiration. Plasma levels of IL-6, IL-10, PGE2, and TNF-α were determined by ELISA using kits provided by the manufacturing company (Biosource International Cytoscreen™) and expressed in pg/ml.
After induction of AP a systemic inflammatory response is initiated and inflammatory mediators can be measured and predict the severity of inflammation. Inflammatory mediators have a very short half-life and the high levels are observed in the first hours of systemic inflammatory response. In a previous publication the authors showed that 2 h is an adequate time to measure the cytokine levels in this experimental model 16.
Cultures and measurement of pancreatic infection
At 24 h after induction of AP, 20 rats from each group were submitted to a midline laparotomy incision under anesthesia and tissue samples were harvested from the pancreas, mesenteric lymph nodes, and liver. All samples were weighed and homogenized and aerobic cultures were made using blood agar plates and MacConkey agar plates and expressed in colony forming units (CFU) per gram. Blood and free peritoneal fluid were sampled using sterile syringes and cultured using an automated culture system (BACTEC PEDS Plus); all positive culture were inoculated on blood agar plates and MacConkey agar plates for bacterial identification.
The presence of pancreatic infection was accepted when CFU/g was >105.
Histological analysis
Fragments of pancreas were harvested and fixed in 10% formaldehyde solution, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) for light microscopy. The inflammatory changes in the pancreas were analyzed and classified by a previous described score 17.
Mortality study
Twenty animals from each group were kept alive for 7 days to determine the mortality rate. These animals were maintained in individual cages, received regular rat chow, and received saline solution or indomethacin according to the experimental protocol.
Statistical analysis
The data were analyzed using Graphpad Prism® version 2.1. Different groups were compared using Fisher's exact test, ANOVA, Tukey, Mann-Whitney, Kruskal-Wallis, and Dunn for the appropriate categorical data. Statistical analyses of the mortality rate were performed using Kaplan–Meier curve and log-rank test. Standard error was used and differences were considered statistically significant when p values were <0.05.
Results
All experiments were successfully concluded.
Cytokine levels
The levels of IL-6, IL-10, PGE2, and TNF-α were undetectable in the sham group. A significantly higher level of cytokines was observed in the pancreatitis group. Significant decreases in levels of IL-6, IL-10, and PGE2 were observed after indomethacin administration when compared with the pancreatitis group (p<0.05). The administration of indomethacin was not able to reduce the level of TNF-α (Table I).
Table I. Cytokine levels 2 h after AP induction in all analyzed groups.
| Group | IL-6 | IL-10 | PGE2 | TNF-α |
|---|---|---|---|---|
| Sham | 0 | 0 | 151±115 | 0 |
| Pancreatitis | 184±19 | 201±34 | 6728±1360 | 175±14 |
| Indomethacin | 74±2* | 47±13* | 306±62* | 155±20 |
Mean value of plasma levels of IL-6, IL-10, PGE2, and TNF-α expressed in pg/ml.
*p<0.05, indomethacin group vs pancreatitis group, ANOVA.
Cultures
Pancreas
Positive cultures were not observed in the sham group. A significantly increased rate of positive bacterial cultures was obtained from pancreas cultures in the pancreatitis and indomethacin groups. There were no significant differences in the incidence of positive cultures between these groups (Table II). A high incidence of Escherichia coli was observed in pancreas cultures in the pancreatitis and indomethacin groups.
Table II. Occurrence of bacterial translocation 24 h after taurocholate-induced pancreatitis.
| Group | Pancreas | Mesenteric lymph nodes | Liver | Blood | Peritoneal cavity |
|---|---|---|---|---|---|
| Sham | 0/10* | 0/10* | 0/10* | 0/10* | 0/10* |
| Pancreatitis | 8/10 | 8/10 | 4/10 | 7/10 | 8/10 |
| Indomethacin | 7/10 | 6/10 | 5/10 | 8/10 | 8/10 |
The occurrence of bacterial translocation was considered positive in all positive cultures.
*p<0.05, sham group vs pancreatitis and indomethacin groups, Fischer test.
Mesenteric lymph nodes
The pancreatitis and indomethacin groups had a significantly increased rate of positive bacterial cultures when compared with the sham group. Positive culture was not observed in the sham group. There were no significant differences in positive cultures and bacterial populations between the pancreatitis and indomethacin groups (Table II).
Liver
A higher incidence of positive cultures was observed in the pancreatitis and indomethacin groups, without significant differences in both groups. Positive culture was not observed in the sham group (Table II).
Blood and peritoneal cavity
A significantly increased rate of positive bacterial cultures was obtained from blood and peritoneal cultures in the pancreatitis and indomethacin groups in contrast with the sham group. There was no significant difference between the pancreatitis and indomethacin groups (Table II).
Pancreatic infection
Infection of pancreatic tissue was not observed in the sham group. A high incidence of pancreatic infection was observed in the pancreatitis group (60%) and was also observed after indomethacin administration (70%) (Figure 1).
Figure 1. .
Percentage of pancreatic infections in rats with taurocholate-induced pancreatitis 24 h after induction of pancreatitis. *p<0.05, sham group vs pancreatitis and indomethacin groups, Fischer test.
Histologic analysis
Pancreatic edema, inflammatory infiltration, pancreatic necrosis, pancreatic hemorrhage, and extrapancreatic fat necrosis were not reduced after indomethacin administration (p>0.05) (Figure 2).
Figure 2. .
Histologic grading of lesions in histological analysis of pancreatic tissue of rats with taurocholate-induced acute pancreatitis. *p<0.05, sham group vs pancreatitis and indomethacin groups, Kruskal-Wallis test.
Mortality study
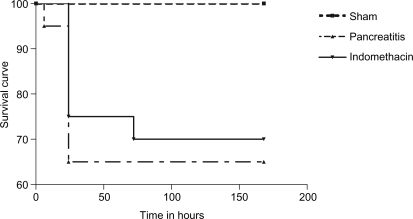
Mortality was not observed in the sham group. Seven rats died after induction of AP in the pancreatitis group (35% mortality rate) and a significant reduction of mortality rate was not observed in the indomethacin group (30% mortality rate) (Figure 3).
Figure 3. .
Kaplan–Meier survival curve of rats with taurocholate-induced pancreatitis in all analyzed groups. p>0.05, pancreatitis group vs indomethacin group, log-rank test.
Discussion
PGE2 has an inflammatory property and its role in AP is unclear. Several investigations have shown that high levels of PGE2 could be responsible for triggering factors of inflammation and suggested that PGE2 inhibition could have beneficial effects in AP 14. Furthermore, experimental studies in burn models described the effects of PGE2 in bacterial translocation, proposing a suppression of intestinal T-cell proliferation allowing bacterial translocation caused by high levels of PGE2 15,18.
All major inflammatory mediators have been studied in recent years and researchers are still looking for the elucidation of the key mediators in severe AP associated with the discovery of specific inhibitors that would make it possible to develop a clinically effective anti-inflammatory therapy 19,20. Trying to answer this question and clarify the role of PGE2 in AP, we used indomethacin as a PGE2 synthesis blocker and correlated with other inflammatory mediators, such as IL-6, IL-10, and TNF-α.
In the present study, the administration of indomethacin successfully inhibited PGE2 production and also decreased levels of IL-6 and IL-10. However, it was not able to reduce TNF-α levels. After administration of indomethacin the levels of TNF-α were kept high in the indomethacin group, demonstrating that indomethacin was incapable of interfering with TNF-α production. Reduction of PGE2 to very low levels in this experimental protocol did not reduce the severity of AP, the incidence of bacterial translocation and pancreatic infection, or the mortality rate.
These data suggest that AP is a very complex inflammatory process involving multiple mediators and that PGE2 does not play a fundamental role in this disease. With respect to the decreased serum levels of IL-6 and IL-10, these data also suggest that isolated reduction of IL-6 and IL-10 was not able to influence the systemic inflammatory response in AP and indicate that TNF-α might play a very important role in the inflammatory process of severe AP and might be a better marker of severity than other inflammatory mediators 21,22,23,24.
In septic burn models, high levels of PGE2 had been correlated with macrophage dysfunction and the administration of a COX inhibitor improved immunity and reduced bacterial translocation 15. Our study did not show any reduction in bacterial translocation and pancreatic infection after indomethacin administration and marked PGE2 inhibition, suggesting that bacterial translocation in AP may have a different and more complex mechanism than those observed in septic-burn models.
The pancreatic infection was identified as the main determinant of morbidity and mortality in severe AP 25,26. In necrotic pancreatic tissue, bacteria find ideal conditions to replicate and form a secondary infectious focus, which could fuel SIRS and increase the mortality rate 27. The present study indicates that pancreatic infection is related to bacterial translocation when associated with AP.
Bacterial translocation is related to mucosal damage caused by hemodynamic and microvascular dysfunction, intravascular thrombosis, vasodilatation, hypotension, and endothelial damage triggered by TNF-α 28. Our present data show high and similar levels of TNF-α in both groups that underwent induction of AP. Based on these data, it is possible to speculate that the intensity of the inflammatory process is mainly related to TNF-α level and this can explain the high occurrence of pancreatic infection and mortality rate in this experimental model 20,22.
It has been reported that PGE2 increases vaso-permeability and sequestration of inflammatory cells 29,30. However, in this model the reduction of PGE2 to very low levels was not able to change the local inflammatory process based on pancreatic histologic analysis. The multiplicity of factors involved in the pathophysiology of AP could explain this finding. Furthermore, the mortality rate in this experimental rat model did not have any beneficial advantage in rats treated with indomethacin. Indeed, it is possible to hypothesize that PGE2 does not play a central role in the physiopathology of AP and PGE2 probably is not an important target for anti-inflammatory therapy in severe AP.
In conclusion, the inhibition of PGE2 production, even when associated with reduction of IL-6 and IL-10 levels, did not have any beneficial effect in this experimental model of AP. Further investigations will be necessary to discover specific inhibitors that would make it possible to develop clinically effective anti-inflammatory therapy in severe AP.
References
- 1.Isenmann R, Rau B, Zoellner U, Beger HG. Management of patients with extended pancreatic necrosis. Pancreatology. 2001;1:63–8. doi: 10.1159/000055794. [DOI] [PubMed] [Google Scholar]
- 2.Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg. 1997;21:130–5. doi: 10.1007/s002689900204. [DOI] [PubMed] [Google Scholar]
- 3.Sakorafas GH, Tsiotou AG. Etiology and pathogenesis of acute pancreatitis: current concepts. J Clin Gastroenterol. 2000;30:343–56. doi: 10.1097/00004836-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Bettinger JR, Grendell JH. Intracellular events in the pathogenesis of acute pancreatitis. Pancreas. 1991;6:2–6. doi: 10.1097/00006676-199101001-00002. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117–25. doi: 10.1002/(SICI)1096-9896(200002)190:2<117::AID-PATH494>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 6.Makhija R, Kingsnorth NA. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:401–10. doi: 10.1007/s005340200049. [DOI] [PubMed] [Google Scholar]
- 7.Foitzik T, Fernandez-Del-Castillo C, Ferraro MJ, Mithofer K, Rattner DW, Warshaw AL. Pathogenesis and prevention of early pancreatic infection in experimental acute necrotizing pancreatitis. Ann Surg. 1995;222:179–85. doi: 10.1097/00000658-199508000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raraty MG, Connor S, Criddle DN, Sutton R, Neoptolemos JP. Acute pancreatitis and organ failure: pathophysiology, natural history, and management strategies. Curr Gastroenterol Rep. 2004;6:99–103. doi: 10.1007/s11894-004-0035-0. [DOI] [PubMed] [Google Scholar]
- 9.Isenmann R, Beger HG. Bacterial infection of pancreatic necrosis: role of bacterial translocation, impact of antibiotic treatment. Pancreatology. 2001;1:79–89. doi: 10.1159/000055798. [DOI] [PubMed] [Google Scholar]
- 10.Thomson A. Bacterial translocation in acute pancreatitis. J Gastroenterol Hepatol. 2003;18:1214. doi: 10.1046/j.1440-1746.2003.03145.x. [DOI] [PubMed] [Google Scholar]
- 11.Souza LJ, Sampietre SN, Figueiredo S, Yria Y, Machado MC, Pinotti HW. Translocação bacteriana na pancreatite aguda. Estudo experimental em ratos. Rev Hosp Clin Fac Med S Paulo. 1996;31:116–20. [PubMed] [Google Scholar]
- 12.Souza LJ, Sampietre SN, Assis RS, Knowles CH, Leite KR, Jancar S, et al. Effect of platelet-activating antagonists (BN-52021, WEB-2170, and BB-882) on bacterial translocation in acute pancreatitis. J Gastrointest Surg. 2001;5:364–70. doi: 10.1016/s1091-255x(01)80063-9. [DOI] [PubMed] [Google Scholar]
- 13.Song AM, Bhagat L, Singh VP, Van Acker GS, Steer ML, Saluja AK. Inhibition of cyclooxygenase-2 (COX-2) ameliorates the severity of pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2002;283:1166–74. doi: 10.1152/ajpgi.00370.2001. [DOI] [PubMed] [Google Scholar]
- 14.Foitzik T, Hotz HG, Hotz B, Wittig F, Buhr HJ. Selective inhibition of cyclooxygenase-2 (COX-2) reduces prostaglandin E2 production and attenuates systemic disease sequel in experimental acute pancreatitis. Hepatogastroenterology. 2003;50:1159–62. [PubMed] [Google Scholar]
- 15.Choudhry MA, Fazal N, Namak SY, Haque F, Ravindranath T, Sayeed MM. PGE2 suppresses intestinal T cell function in thermal injury: a cause of enhanced bacterial translocation. Shock. 2001;16:183–8. doi: 10.1097/00024382-200116030-00003. [DOI] [PubMed] [Google Scholar]
- 16.Machado MC, Coelho AM, Pontieri V, Sampietre SN, Molan NA, Soriano F, et al. Local and systemic effects of hypertonic solution (NaCl 7.5%) in experimental acute pancreatitis. Pancreas. 2006;32:80–6. doi: 10.1097/01.mpa.0000191645.01926.8f. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, et al. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukushima R, Alexander JW, Mao WJX, Szczur K, Stephens AM, Ogle JD, et al. Time course of production of cytokines and prostaglandin E2 by macrophages isolated after thermal injury and bacterial translocation. Circ Shock. 1994;42:154–62. [PubMed] [Google Scholar]
- 19.Mayer J, Rau B, Gansauge F, Beger HG. Inflammatory mediators in human acute pancreatitis: clinical and pathophysiology implications. Gut. 2000;47:546–52. doi: 10.1136/gut.47.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirota M, Nozawa F, Okabe A, Shibata M, Beppu T, Shimada S, et al. Relationship between plasma cytokine concentration and multiple organ failure in patients with acute pancreatitis. Pancreas. 2000;21:141–6. doi: 10.1097/00006676-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia M, Neoptolemos JP, Slavin J. Inflammatory mediators as therapeutic targets in acute pancreatitis. Curr Opin Invest Drugs. 2001;2:496–501. [PubMed] [Google Scholar]
- 22.Formela LJ, Galloway SW, Kingsnorth NA. Inflammatory mediators in acute pancreatitis. Br J Surg. 1995;82:6–13. doi: 10.1002/bjs.1800820105. [DOI] [PubMed] [Google Scholar]
- 23.Brivet FG, Emilie D. Predictive value of cytokines during acute severe pancreatitis. Crit Care Med. 2000;28:2673. doi: 10.1097/00003246-200007000-00094. [DOI] [PubMed] [Google Scholar]
- 24.Rongine AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960–7. doi: 10.1053/gast.1997.v112.pm9041259. [DOI] [PubMed] [Google Scholar]
- 25.Samuel S, Lanig S, Lux A, Keese M, Gretz N, Nichterlein T, et al. The gut origin of bacterial pancreatic infection during acute experimental pancreatitis in rats. Pancreatology. 2002;2:449–55. doi: 10.1159/000064714. [DOI] [PubMed] [Google Scholar]
- 26.Pooran N, Indaram A, Singh P, Bank S. Cytokines (IL-6, IL-8, TNF): early and reliable predictors of severe acute pancreatitis. J Clin Gastroenterol. 2003;37:263–6. doi: 10.1097/00004836-200309000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Reber HA. Pathogenesis of infection in pancreatic inflammatory disease. Pancreatology. 2001;1:207–9. doi: 10.1159/000055811. [DOI] [PubMed] [Google Scholar]
- 28.Foitzik T. The enteral factor in pancreatic infection. Pancreatology. 2001;1:217–23. doi: 10.1159/000055814. [DOI] [PubMed] [Google Scholar]
- 29.Yucel K, Alhan E, Kucuktulu U, Piri M, Ercin C, Deger O. The effects of prostaglandin E1 on the microperfusion of the pancreas during acute necrotizing pancreatitis in rats. Hepatogastroenterology. 2002;49:544–8. [PubMed] [Google Scholar]
- 30.Yan WW, Zhou ZG, Chen YD, Gao HK. Role of COX-2 I microcirculatory disturbance in experimental pancreatitis. World J Gastroenterol. 2004;10:2095–8. doi: 10.3748/wjg.v10.i14.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]