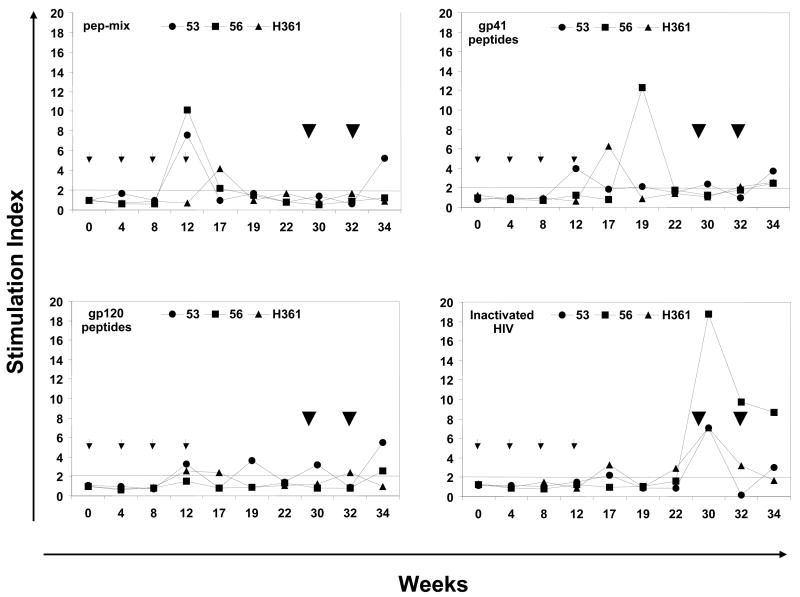
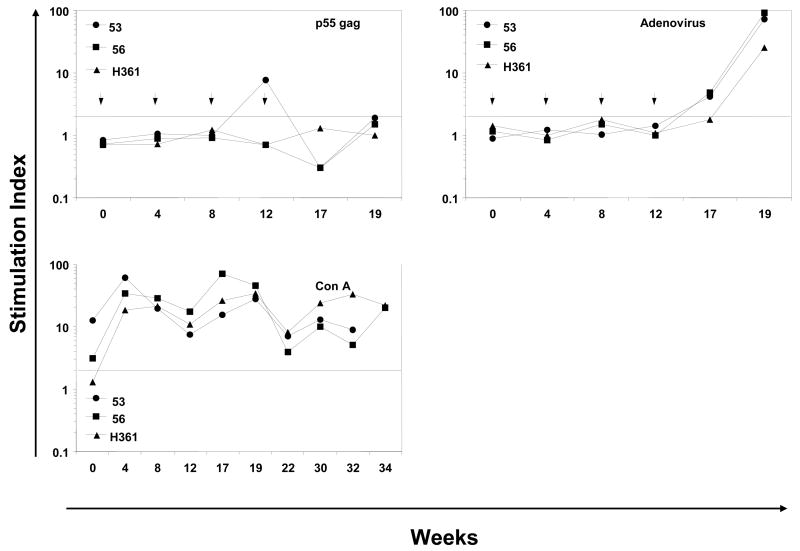
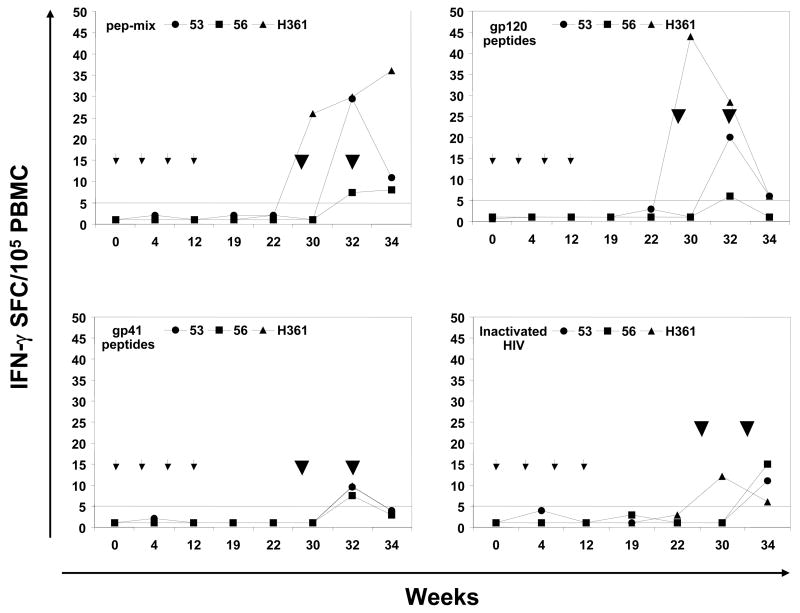
Figure 4.
Antigen-specific cellular immune responses. The proliferative responses in terms of [3H] thymidine incorporation and IFN-γ producing cells by the ELISPOT method were determined using PBMC samples from rhesus macaques immunized by oral priming with enteric-coated capsules delivering Ad-gag and Ad-env-peptide and intranasal boosting with synthetic HIV-1 envelope peptides and CT2*, the mutant cholera toxin adjuvant. (A) Systemic T cell responses using PBMC were determined in terms of proliferation in response to mixtures of all six HIV-1 envelope peptides (pep-mix), two gp41 peptides, four gp120 peptides, and heat-inactivated HIV-1IIIB preparation. The fold-increases in proliferation responses to the different antigens were calculated by comparing the values from cells in the culture medium. (B) Proliferation responses of PBMC were determined in response to recombinant HIV-1 gag protein (p55 gag), heat-inactivated Ad5, and Con A. (C) ELISPOT analyses for antigen-specific IFN-γ-producing cells for 105 input PBMC in each monkey, in terms of spot-forming-cells (SFC/105 PBMC) at the indicated times after oral Ad vector mediated priming and intranasal boosting with peptide-cocktail (6 peptides, 100 μg each/dose) mixed with mutant cholera toxin, CT2* (10 μg/dose). The horizontal line in each panel indicates the cut-off value for positive response (SI of 2.0 for the proliferation assay and SFC of 5 for the ELISPOT assay).