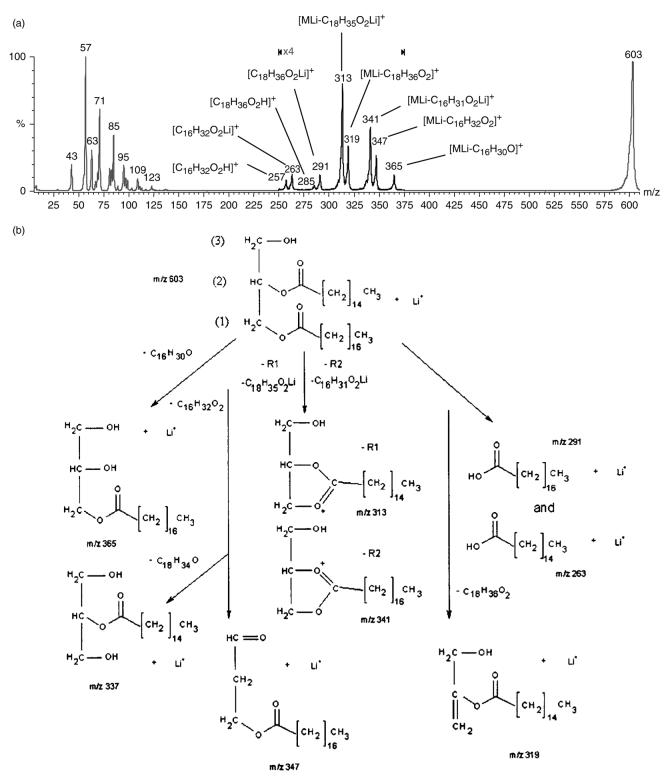
Figure 5.
(a) Product ion spectrum of 1-stearin-2-palmitin standard at m/z 603. The greater peak intensity of m/z 313 vs m/z 341 allows the identification of the 1-position of the 1,2-diglyceride. (b) Major fragment ions produced from the CID of 1-stearin-2-palmitin m/z 603 [MLi]+: the m/z 313 ion from the neutral loss of C18 : 0 lithium fatty acetate (1-position), [MLi – C18H35O2Li]+, the ion at m/z 341 from the neutral loss of C16 : 0 lithium fatty acetate (2-position), [MLi – C16H31O2Li]+, the m/z 365 ion from the loss of the C16 : 0 fatty acyl chain as ketene [MLi – C16H30O]+, the neutral loss of C16 : 0 fatty acid at m/z 347 [MLi – C16H32O2]+, the neutral loss of the C18 : 0 fatty acid at m/z 319 [MLi – C18H36O2]+, the lithium adducts of stearic acid at m/z 291 [C18H36O2Li]+, the lithium adduct of palmitic acid at m/z 263 [C16H32O2Li]+ and protonated stearic and palmitic acids at m/z 285 [C18H36O2H]+ and m/z 257 [C16H32O2H]+, respectively.