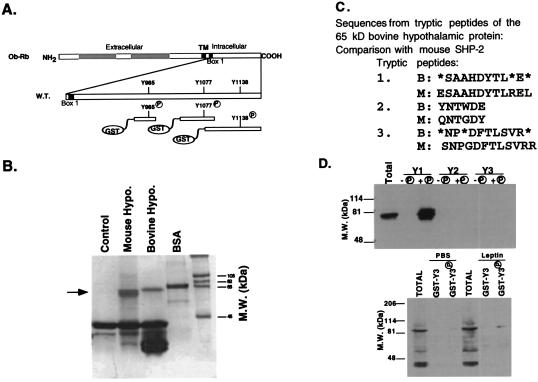
Figure 2.
Binding of a 64-kDa protein to phosphotyrosine 985 from the Ob-Rb cytoplasmic region. (A) The structure of three receptor-GST constructs, each containing an individual tyrosine from the cytoplasmic domain of Ob-Rb, is shown. (B) The GST fusion protein with phosphorylated Tyr985 binds to a 64-kDa protein in mouse and bovine hypothalamus. The GST-Ob-Rb peptide spanning Tyr 985 was incubated with protein extracts from bovine or mouse hypothalamus. After precipitation, bound proteins were eluted, were resolved on SDS/PAGE, and were stained with Coomassie blue. A 64-kDa protein was observed. (C) Microsequencing of three internal tryptic fragments of the 64-kDa protein yielded peptide sequences identical to that of SHP-2, SH2 domain-containing protein tyrosine phosphatase 2. (D) Immunoblots of the protein precipitate of Ob-Rb fragments. The phosphorylated and unphosphorylated GST fusion proteins for fragments 1, 2, and 3 were incubated with protein extracts of mouse or bovine hypothalamus. The precipitated proteins were immunoblotted by using specific antibodies to SHP-2. When extracts from PBS- or leptin-treated mice were incubated with GST fusion proteins for fragment 3, STAT3 was detected.