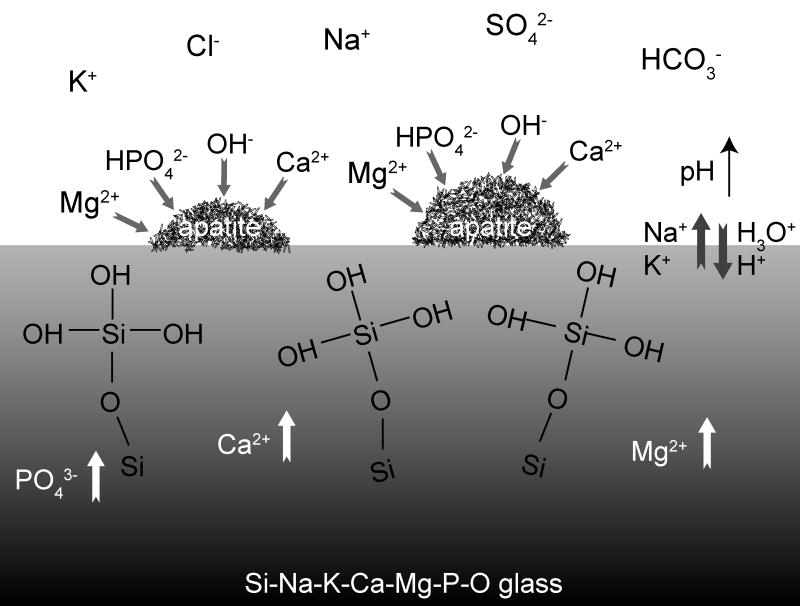
Figure 4.
The mechanisms of reaction of the Si-Na-K-Ca-Mg-P-O glasses with SBF are consistent with those described by Hench for Bioglass® [20,37,38]. The steps involved are: the exchange of Na+ and K+ from the glass with H+ or H3O+ from solution, accompanied by the loss of soluble silica into the solution and the formation of silanols on the glass surface; condensation and repolymerization of a SiO2-rich layer on the surface; migration of Ca2+ and PO3-4 through the silica-rich layer forming a CaO-P2O5-rich film that incorporates calcium and phosphates from solution; finally, the crystallization of the amorphous calcium phosphate film to form an apatite layer. De Aza et al. [39] have pointed out that the increase in pH on the glass surface due to the ionic exchange between the labile cations Na+, K+, Ca+ etc., is necessary for the partial dissolution of the silica-rich layer and the subsequent apatite precipitation.