Abstract
Shewanella oneidensis MR-1 grew for over 50 days in microbial fuel cells, incompletely oxidizing lactate to acetate with high recovery of the electrons derived from this reaction as electricity. Electricity was produced with lactate or hydrogen and current was comparable to that of electricigens which completely oxidize organic substrates. However, unlike fuel cells with previously described electricigens, in which cells are primarily attached to the anode, at least as many of the S. oneidensis cells were planktonic as were attached to the anode. These results demonstrate that S. oneidensis may conserve energy for growth with an electrode serving as an electron acceptor and suggest that multiple strategies for electron transfer to fuel cell anodes exist.
Keywords: Shewanella, microbial fuel cell, biofilm, anaerobic respiration
Introduction
There is a compelling need to identify pure cultures that can serve as appropriate models for electron transfer to the anodes of microbial fuel cells because little is known about the physiology of this process. Shewanella species were the first organisms proposed to transfer electrons to the surface of electrodes via electron-transfer proteins (Kim et al., 1999a, b, 1999). This, coupled with the relative ease in growing these organisms, has led to their common use as model organisms for the study of microbial fuel cells (Kim et al., 2002; Park & Zeikus, 2002; Gorby et al., 2006; Ringeisen et al., 2006; Biffinger et al., 2007; Cho & Ellington, 2007).
However, little is known about the physiology of Shewanella species growing with anodes serving as the sole electron acceptor. Most studies (Kim et al., 2002; Park & Zeikus, 2002; Cho & Ellington, 2007) have evaluated electricity production in short-term studies with cell suspensions. Cell suspension studies do not give appropriate information on the long-term functioning of microbial fuel cells that is necessary for applications (Shukla et al., 2004; Lovley, 2006). As previously discussed (Lovley, 2006), in the one previous study designed to evaluate Shewanella growth with an electrode serving as the electron acceptor (Kim et al., 1999a, b, 1999), growth was not directly coupled to electricity production and a low percentage (<10%) of the electrons available in the electron donor (lactate) was recovered as current. The possibility that the cultures might have been contaminated (Kim et al., 2002) further complicates analysis of the data. In a more recent study, which was designed to optimize power output of a growing culture of Shewanella oneidensis, electron recovery as current remained <10% (Ringeisen et al., 2006).
In order to learn more about the long-term sustainability of Shewanella-based microbial fuel cells, the growth of the most commonly studied strain of the Shewanella genus, S. oneidensis MR-1, was investigated. The results demonstrate that mechanisms for growth and electron transfer with electrodes serving as the electron acceptor in S. oneidensis are different than the patterns observed in electricigens, microorganisms that completely oxidize organic fuels with direct electron transfer to the anode surface.
Experimental
Growth conditions and microbial fuel cells
Shewanella oneidensis strain MR-1 (ATCC 7005500), was grown in anaerobic, freshwater, lactate (20 mM)-Fe(III) citrate (50 mM) medium (FW medium) as described previously (Lovley et al., 1989) with the exception that the three amino acids typically added to the medium (l-arginine, l-glutamine, and dl-serine) were deleted and the medium was amended with 0.05% (w/v) yeast extract. The cells from a 100 mL culture near the end of log phase were collected by centrifugation and resuspended in FW medium (5 mL) without yeast extract or Fe(III) citrate. These cells (3.7 mg of total protein) were inoculated into the anode chamber of previously described (Bond & Lovley, 2003) fuel cells in which graphite sticks (surface area 61.2 cm2) serve as the anode and cathode. The electrical connection between the anode and cathode included a resistor of 560 Ω (Bond & Lovley, 2003). Current was measured hourly with a Keithley Model 2700 Digital Multimeter (Keithley Instruments, Cleveland, OH). The anode chamber contained 225 mL of FW medium described above or ‘defined FW medium’ in which the yeast extract was omitted and substituted with l-arginine (22 mg L−1), l-glutamine (22 mg L−1), and dl-serine (44 mg L−1). The initial lactate concentration was 10 mM. The cathode chamber contained 225 mL of FW medium without yeast extract, amino acids, or Fe(III). The anode chamber was continuously bubbled with N2/CO2 (80 : 20) and the cathode chamber was continuously bubbled with air. All incubations were at 30 °C.
Protein quantification
Total protein content from planktonic cells in the anode chambers was quantified after each exchange of the medium and after termination of the experiments. Protein concentration was determined on centrifuged cell pellets and on biofilm scraped from electrodes with sterile razorblades by the bicinchoninic acid method with bovine serum albumin as a standard (Smith et al., 1985).
Microscopy
Cells associated with the anodes were stained with the BacLight Live/Dead viability kit (Invitrogen–Molecular Probes Inc., Eugene, OR), as described previously (Korber et al., 1996) before examination with confocal laser scanning microscopy (CLSM). Samples were examined on a Zeiss Axiovert LSM 510 Meta Confocal System equipped with a 63 × Zeiss plan-apochromat oil immersion objective (numerical aperture of 1.4) and a Meta detector (Carl Zeiss MicroImaging Inc., Thornwood, NY). The confocal microscope was equipped with an argon laser (lines at 458, 477, 488 and 514 nm; 25 mW total), and two HeNe lasers (543 nm, 1 mW; 633 nm, 5 mW), krypton–argon dual laser (488 and 568 nm) and a diode laser (638 nm). Representative three-dimensional scans of biofilm sections on the electrodes were taken, and displayed as ortho view. Images were averaged by Kalman filtration with eight running scans per image (Manz et al., 1999). The acquisition software was lsm 510 meta, version 3.2 SP2. The ratio of live/dead cells on the anodes was determined by quantifying the ratio of dead cells (red) and live cells (green) from confocal images as described previously (Lanthier et al., 2005). The imagej software v1.31 (US National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/) was used for image analysis. Anodes sacrificed for SEM were prepared as described previously (Bond & Lovley, 2003) except that the whole electrode was chemically dehydrated with hexamethyldisilazane.
Results and discussion
Current production
With either the yeast extract-amended or defined medium, current production increased over time to a maximum of 0.2–0.3 mA (Fig. 1a and b). Maximum current was typically reached earlier in the medium containing yeast extract than the defined medium, but there was no consistent difference in the maximum current between the two medium types (Fig. 1). The anode chambers were turbid with planktonic cells and higher turbidity was observed with yeast extract-amended medium (see biomass quantification by protein assay in the next section). The medium in the anode chambers was exchanged by removing the medium and planktonic cells (but retaining anode-associated cells) and replacing with new FW medium (Bond & Lovley, 2003). After addition of 3 mM lactate, current production resumed at levels comparable (0.2–0.3 mA) to those before exchanging the medium within 12 h in defined medium and within 3 h in medium containing yeast extract. This observation was consistent after each exchange of the medium (Fig. 1a and b).
Fig. 1.
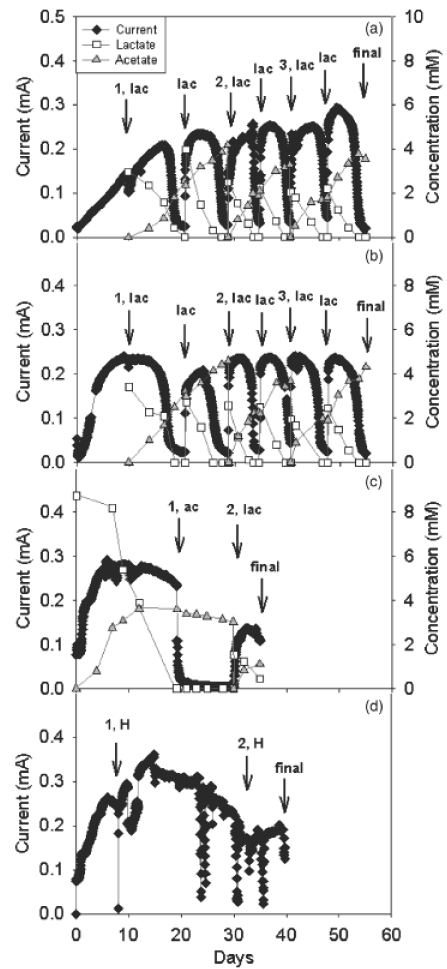
Current production, lactate consumption and acetate accumulation by Shewanella oneidensis MR-1 growing with lactate as an electron donor and an anode as the sole terminal electron acceptor in (a) defined FW medium; (b) FW medium supplemented with 0.05% yeast extract. Fuel cells fed with acetate in defined FW medium (c) or hydrogen in defined FW medium (d) were first started with lactate as an electron donor, and medium was exchanged with the appropriate electron donor when current production was steady. First (1), second (2) or third (3) medium exchange in the anode chamber was accompanied by addition of 3 mM lactate (lac), 4 mM acetate (ac), or bubbling with hydrogen (as H2/CO2/N2) (H), as noted. Sampling of medium and electrode at the end of experiment was dubbed ‘final’ (final). The medium in fuel cell C was only exchanged twice.
Addition of yeast extract did not increase current production, but increased protein biomass both in suspension and on the anode (Fig. 2). Current was not observed in the absence of cells (data not shown), or in the presence of 4 mM acetate (Fig. 1c), an electron donor not utilized by S. oneidensis under anaerobic conditions (Lovley et al., 1992). When the acetate medium was exchanged with medium containing lactate, power production resumed, but at a somewhat lower initial rate (Fig. 1c). This is presumably due to the inability of cells to maintain as active a population during the incubation with acetate.
Fig. 2.
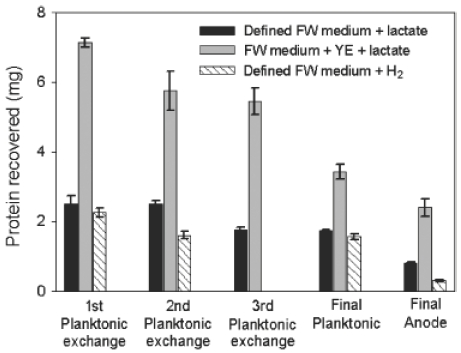
Protein content of the medium in the anode chamber and on the anode of fuel cells inoculated with Shewanella oneidensis MR-1. Protein from planktonic cells was determined after each exchange of the medium. Protein from both planktonic and anode associated cells was determined after termination of the experiment. All fuel cells contained FW medium supplemented with lactate, except the fuel cell with hydrogen, which was supplemented with lactate and no hydrogen until the first medium exchange, and then bubbled with hydrogen without lactate. No third medium exchanged was performed on the fuel cell with defined medium and hydrogen.
In order to ensure that the current production in the presence of cells and lactate was not limited by reactions at the cathode, similar studies were also conducted in which the anode was poised with a potentiostat at +300 mV (vs. Ag/AgCl reference) as described previously (Bond & Lovley, 2003). However, sustained power production in the poised system was no higher than in fuel cells (data not shown). Therefore, all remaining studies were conducted as fuel cells.
Growth and electron recovery
The protein content from planktonic cells in the anode chambers was quantified after each exchange of the medium and after termination of the experiments (as indicated in Fig. 1). Protein on the anode was determined only after termination of the experiments (Fig. 1). There were substantial amounts of planktonic protein biomass in the anode chambers with higher quantities of planktonic protein biomass in the yeast-extract amended medium than the defined medium (Fig. 2). Planktonic cells became abundant each time the anode medium was exchanged, as indicated by the return of visible turbidity and measured by high planktonic cell protein after each medium exchange (Fig. 2). Planktonic protein biomass declined with each medium exchange accompanied by lactate amendment. The decrease in planktonic protein biomass over the course of the experiment was not as large in defined medium as in yeast extract amended medium. After multiple medium replacements (Fig. 1a, b and d), the anodes were removed from the anode chamber and analyzed for protein. There was less attached protein biomass than planktonic protein biomass at the termination of the experiment (Fig. 2). There was more protein biomass on the anodes from yeast extract medium than defined medium.
Growth of MR-1 with the anode serving as the sole electron acceptor was further evaluated by inoculating a fuel cell containing defined FW medium and 10 mM lactate with a small inoculum of cells (0.04 mg protein). After 10 days, current was produced at a level equivalent to that observed with the larger inocula shown in Fig. 1. After 14 days, the total protein in the anode chamber and on the anode surface was 2.29 mg (anode: 0.28 mg). These results demonstrate that S. oneidensis may conserve energy to support growth using the anode in a fuel cell as the sole terminal electron acceptor.
Growth on the anodes was also apparent with CLSM and SEM (Fig. 3). Cells were scattered over the surface of the anodes from fuel cells with defined medium (Fig. 3a and b). There was greater coverage of the anodes from fuel cells with yeast extract medium, but there were still substantial portions of the anode that were not colonized with cells and in no instance were thick biofilms of multiple stacked cells observed (Fig. 3c and d). CLSM images indicate a higher density of live cells on the anode recovered from the fuel cell amended with yeast extract (97.0±3.5%; n = 7) than in defined medium (75.2±7.2%; n = 7), further suggesting that the yeast extract-amended medium was a superior medium for growth in fuel cells. These anaerobic anode biofilms differ significantly from the much more extensive biofilms that S. oneidensis MR-1 forms when grown in aerobic flow-through cells with rich medium (Thormann et al., 2004).
Fig. 3.
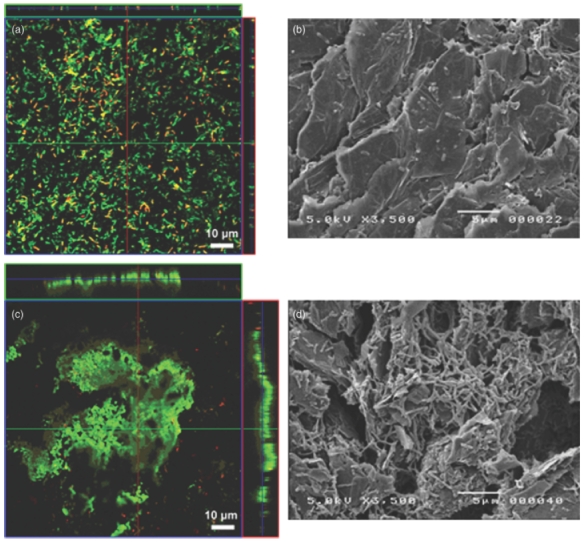
Confocal (a, c) and SEM (b, d) pictures of the biofilm growing on anodes of fuel cells inoculated with Shewanella oneidensis MR-1. Fuel cells were fed with FW medium supplemented with lactate and (a, b) amino acids; (c, d) yeast extract. Anodes for CLSM examination were directly stained with the BacLight Live/Dead kit (Korber et al., 1996). Live cells appear green and dead cells red.
Analysis of the stoichiometry of lactate metabolism and electricity production demonstrated that lactate was consumed over time with an approximate (1 : 1) stoichiometric accumulation of acetate (Fig. 1a and b). The medium was exchanged after every second addition of lactate in the microbial fuel cells. For the first additions of lactate after the medium was exchanged, the accumulation of acetate from lactate was 86.8±1.6% (mean±SD; n = 3) in the defined medium, whereas in the yeast extract-amended medium the recovery was 94.9±5.4%. These results show that lactate was partially oxidized by MR-1 according to the reaction
which is consistent with previous studies (Lovley et al., 1989, 1992).
The coulombic yield based on partial lactate oxidation (4 e− mol−1 lactate) was 55.9±12.9% (n = 3) in defined medium and 56.2±14.8% for the medium amended with yeast extract. In this case, a coulombic yield less than 100% reflects the fraction of electrons obtained from partial lactate oxidation which were diverted to cell synthesis, rather than electricity production, during growth of MR-1 on the anode. Similar electron recovery was observed with MR-1 during growth on nitrate (Middleton et al., 2003) and are typical of other bacteria which exhibit anaerobic nonfermentative respiration and growth (Löffler et al., 1999).
In order to evaluate the potential for electricity production with hydrogen as the electron donor, cells were initially inoculated into an anode chamber containing defined lactate medium and once current production was established, the medium was replaced with lactate-free medium and continuously bubbled with a mixture of H2/CO2/N2 (7 : 10 : 83). Current production was comparable to that with lactate as the electron donor (Fig. 1d). As in lactate cultures, there was visible turbidity in the anode chamber, corresponding with the recovery of cell protein in the medium (Fig. 2). Turbidity decreased after the media was exchanged with lactate-free medium, but remained stable each time the medium was exchanged afterward (Fig. 2). There was less protein biomass attached to the anode than planktonic protein biomass, consistent with the results with lactate as the fuel (Fig. 2).
Conclusion
These results further demonstrate the diversity of strategies that microorganisms may employ for growth with electrodes serving as the electron acceptor. For example, in previous studies with other pure cultures (Bond & Lovley, 2003, 2005; Chaudhuri & Lovley, 2003; Holmes et al., 2004a, b), the electricity-producing microorganisms were predominately attached to the anode surface. In contrast, most of the cellular protein in the S. oneidensis fuel cells presented in this study were planktonic. Shewanella species are capable of producing electron shuttles, which permit them to reduce insoluble Fe(III) oxides without direct cell-electron acceptor contact (Newman & Kolter, 2000; Nevin & Lovley, 2002a, b; Lies et al., 2005). Although these electron shuttles are generally considered to be small, soluble molecules, they might also be comprised of the membrane vesicles containing redox proteins that are known to be released from gram-negative microorganisms (Krause et al., 1996; Schooling & Beveridge, 2006). It seems likely that electron shuttle production permits S. oneidensis to oxidize lactate or hydrogen without being attached to the anode surface as electrochemical analyses suggest electron-shuttling vs. direct electron transfer in Shewanella-based microbial fuel cells (Marsili et al., 2007). For some organisms, such as Geobacter species, which do not produce electron shuttles (Nevin & Lovley, 2000; Bond & Lovley, 2003) close association of the cells with the anode is likely to be essential for electron transfer (Bond & Lovley, 2003; Reguera et al., 2006). However, even Geothrix fermentans, which produces an electron shuttle (Nevin & Lovley, 2002a, b) remains affixed to the anode surface in microbial fuel cells (Bond & Lovley, 2005).
Incomplete oxidation of lactate to acetate coupled to electron transfer to electrodes similar to that reported here for S. oneidensis, was observed previously in Geothrix fermentans (Bond & Lovley, 2005). However, G. fermentans subsequently oxidized the acetate whereas S. oneidensis cannot. This is an important consideration because this means that only a third of the electrons available in the initial fuel is converted to electricity.
Although the ability of Shewanella species to produce electricity from lactate has been noted many times (Kim et al., 1999a, b, 1999, 2002; Ringeisen et al., 2006; Biffinger et al., 2007; Cho & Ellington, 2007), the results presented here demonstrate for the first time that a substantial proportion of the electrons derived from lactate oxidation by S. oneidensis can be recovered as electrons in a microbial fuel cell. This is an important consideration because electron recoveries of <1% (Kim et al., 2002) and 10% (Ringeisen et al., 2006), are difficult to reconcile with the concept that Shewanella species may use electrodes as a respiratory electron acceptor.
The possibility of producing electricity with microbial cultures has been known for a long time, the study of self-sustaining microbial fuel cells in which microorganisms, known as electricigens, conserve energy to support growth from electron transfer to electrodes is in its infancy (Lovley, 2006). To date, each pure culture study with electricigens has demonstrated that the particular organism under investigation has properties in fuel cells that are significantly different than previously studied organisms (Bond et al., 2002; Bond & Lovley, 2003, 2005; Chaudhuri & Lovley, 2003; Holmes et al., 2004a, b; Biffinger et al., 2007). The finding that S. oneidensis offers yet another variation on growth on electrodes emphasizes the need to further evaluate the diversity of microorganisms capable of respiration with electrodes in order to realize the full range of microbial strategies that can be potentially exploited for the optimization of microbial fuel cells.
Acknowledgments
This research was supported by the Office of Naval Research Award No. N00014-06-1-0802 and the Office of Science (BER), US Department of Energy, Grant No. DE-FC02-02ER63446.
Statement
OnlineOpen articles are made available in accordance with the terms of the Creative Commons Deed, Attribution 2.5 (further details available from http://www.creativecommons.org), which allows Open Access dissemination of the article, but does not permit commercial exploitation or the creation of derivative works.
References
- Biffinger JC, Pietron J, Ray R, Little B, Ringeisen BR. A biofilm enhanced miniature microbial fuel cell using Shewanella oneidensis DSP10 and oxygen reduction cathodes. Biosens Bioelec. 2007;22:1672–1679. doi: 10.1016/j.bios.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Bond DR, Lovley DR. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol. 2003;69:1548–1555. doi: 10.1128/AEM.69.3.1548-1555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond DR, Lovley DR. Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans. Appl Environ Microbiol. 2005;71:2186–2189. doi: 10.1128/AEM.71.4.2186-2189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond DR, Holmes DE, Tender LM, Lovley DR. Electrode-reducing microorganisms that harvest energy from marine sediments. Science. 2002;295:483–485. doi: 10.1126/science.1066771. [DOI] [PubMed] [Google Scholar]
- Chaudhuri SK, Lovley DR. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat Biotechnol. 2003;21:1229–1232. doi: 10.1038/nbt867. [DOI] [PubMed] [Google Scholar]
- Cho EJ, Ellington AD. Optimization of the biological component of a bioelectrochemical cell. Bioelectrochem. 2007;70:165–172. doi: 10.1016/j.bioelechem.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Gorby YA, Yanina S, McLean JS, et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci USA. 2006;103:11358–11363. doi: 10.1073/pnas.0604517103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes DE, Bond DR, Lovley DR. Electron transfer by Desulfobulbus propionicus to Fe(III) and graphite electrodes. Appl Environ Microbiol. 2004a;70:1234–1237. doi: 10.1128/AEM.70.2.1234-1237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes DE, Nicoll JS, Bond DR, Lovley DR. Potential role of a novel psychrotolerant member of the family Geobacteraceae, Geopsychrobacter electrodiphilus gen. nov., sp nov., in electricity production by a marine sediment fuel cell. Appl Environ Microbiol. 2004b;70:6023–6030. doi: 10.1128/AEM.70.10.6023-6030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BH, Ikeda T, Park HS, et al. Electrochemical activity of an Fe(III)-reducing bacterium, Shewanella putrefaciens IR-1, in the presence of alternative electron acceptors. Biotechnol Tech. 1999a;13:475–478. [Google Scholar]
- Kim BH, Kim HJ, Hyun MS, Park DH. Direct electrode reaction of Fe(III)-reducing bacterium, Shewanella putrefaciens. J Microbiol Biotechnol. 1999b;9:127–131. [Google Scholar]
- Kim HJ, Hyun MS, Chang IS, Kim BH. A microbial fuel cell type lactate biosensor using a metal-reducing bacterium, Shewanella putrefaciens. J Microbiol Biotechnol. 1999;9:365–367. [Google Scholar]
- Kim HJ, Park HS, Hyun MS, Chang IS, Kim M, Kim BH. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzyme Microb Technol. 2002;30:145–152. [Google Scholar]
- Korber DR, Choi A, Wolfaardt GM, Caldwell DE. Bacterial plasmolysis as a physical indicator of viability. Appl Environ Microbiol. 1996;62:3939–3947. doi: 10.1128/aem.62.11.3939-3947.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause B, Beveridge TJ, Remsen CC, Nealson KH. Structure and properties of novel inclusions in Shewanella putrefaciens. FEMS Microbiol Lett. 1996;139:63–69. doi: 10.1111/j.1574-6968.1996.tb08180.x. [DOI] [PubMed] [Google Scholar]
- Lanthier M, Juteau P, Lépine F, Beaudet R, Villemur R. Desulfitobacterium hafniense is present in a high proportion within the biofilms of a high-performance pentachlorophenol-degrading, methanogenic fixed-film reactor. Appl Environ Microbiol. 2005;71:1058–1065. doi: 10.1128/AEM.71.2.1058-1065.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lies DP, Hernandez ME, Kappler A, Mielke RE, Gralnick JA, Newman DK. Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for biofilms. Appl Environ Microbiol. 2005;71:4414–4426. doi: 10.1128/AEM.71.8.4414-4426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffler FE, Tiedje JM, Sanford RA. Fraction of electrons consumed in electron acceptor reduction and hydrogen thresholds as indicators of halorespiratory physiology. Appl Environ Microbiol. 1999;65:4049–4056. doi: 10.1128/aem.65.9.4049-4056.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley DR. Bug juice: harvesting electricity with microorganisms. Nature Rev Microbiol. 2006;4:497–508. doi: 10.1038/nrmicro1442. [DOI] [PubMed] [Google Scholar]
- Lovley DR, Phillips EJP, Lonergan DJ. Hydrogen and formate oxidation coupled to dissimilatory reduction of iron or manganese by Alteromonas putrefaciens. Appl Environ Microbiol. 1989;55:700–706. doi: 10.1128/aem.55.3.700-706.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley DR, Phillips EJP, Caccavo F. Acetate oxidation by dissimilatory Fe(III) reducers. Appl Environ Microbiol. 1992;58:3205–3206. doi: 10.1128/aem.58.9.3205-3208.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz W, Wendt-Potthoff K, Neu TR, Szewzyk U, Lawrence JR. Phylogenetic composition, spatial structure, and dynamics of lotic bacterial biofilms investigated by fluorescent in situ hybridization and confocal laser scanning microscopy. Microb Ecol. 1999;37:225–237. doi: 10.1007/s002489900148. [DOI] [PubMed] [Google Scholar]
- Marsili E, Baron DB, Bond DR. Techniques for electrochemical characterization of electricity-producing bacteria; 107th General Meeting of the American Society for Microbiology; Toronto, ON: 2007. [Google Scholar]
- Middleton SS, Latmani RB, Mackey MR, Ellisman MH, Tebo BM, Criddle CS. Cometabolism of Cr(VI) by Shewanella oneidensis MR-1 produces cell-associated reduced chromium and inhibits growth. Biotechnol Bioengineer. 2003;83:627–637. doi: 10.1002/bit.10725. [DOI] [PubMed] [Google Scholar]
- Nevin KP, Lovley DR. Lack of production of electron-shuttling compounds or solubilization of Fe(III) during reduction of insoluble Fe(III) oxide by Geobacter metallireducens. Appl Environ Microbiol. 2000;66:2248–2251. doi: 10.1128/aem.66.5.2248-2251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin KP, Lovley DR. Mechanisms for Fe(III) oxide reduction in sedimentary environments. Geomicrobiol J. 2002a;19:141–159. [Google Scholar]
- Nevin KP, Lovley DR. Mechanisms for accessing insoluble Fe(III) oxide during dissimilatory Fe(III) reduction by Geothrix fermentans. Appl Environ Microbiol. 2002b;68:2294–2299. doi: 10.1128/AEM.68.5.2294-2299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DK, Kolter R. A role for excreted quinones in extracellular electron transfer. Nature. 2000;405:94–97. doi: 10.1038/35011098. [DOI] [PubMed] [Google Scholar]
- Park DH, Zeikus JG. Impact of electrode composition on electricity generation in a single-compartment fuel cell using Shewanella putrefaciens. Appl Microbiol Biotechnol. 2002;59:58–61. doi: 10.1007/s00253-002-0972-1. [DOI] [PubMed] [Google Scholar]
- Reguera G, Nevin KP, Nicoll JS, Covalla SF, Woodard TL, Lovley DR. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl Environ Microbiol. 2006;72:7345–7348. doi: 10.1128/AEM.01444-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringeisen BR, Henderson E, Wu PK, et al. High power density from a miniature microbial fuel cell using Shewanella oneidensis DSP10. Environ Sci Technol. 2006;40:2629–2634. doi: 10.1021/es052254w. [DOI] [PubMed] [Google Scholar]
- Schooling SR, Beveridge TR. Membrane vesicles: an overlooked component of the matrices of biofilms. J Bacteriol. 2006;188:5945–5957. doi: 10.1128/JB.00257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla AK, Suresh P, Berchmans S, Rajendran A. Biological fuel cells and their applications. Curr Sci. 2004;87:455–468. [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Thormann KM, Saville RM, Shukla S, Pelletier DA, Spormann AM. Initial phases of biofilm formation in Shewanella oneidensis MR-1. J Bacteriol. 2004;186:8096–8104. doi: 10.1128/JB.186.23.8096-8104.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


