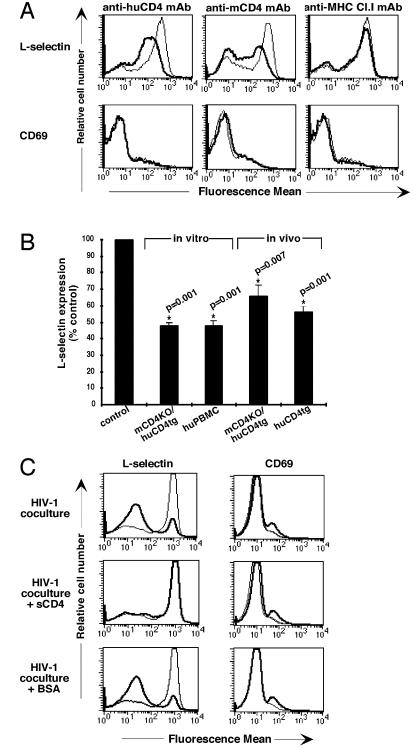
Figure 1.
Down-regulation of l-selectin after in vitro and in vivo ligation of CD4 by mAb or HIV-1. (A) The mouse or human CD4 or MHC class I receptors on enriched CD4+ T cells of mCD4KO/huCD4tg or C57BL/6 mice were crosslinked in vitro as described. After 4 h, cells were harvested and analyzed by flow cytometry. Thin lines represent l-selectin expression or CD69 expression on untreated cells; thick lines represent l-selectin expression or CD69 expression on anti-CD4 or anti-MHC class I mAb-treated cells. Results are representative of at least five experiments. (B) The CD4 receptors on lymphocytes of mCD4KO/huCD4tg mice or human PBMCs were crosslinked in vitro as described in Materials and Methods. For in vivo studies, mCD4KO/huCD4tg and huCD4tg mice were injected with 100 μg of anti-huCD4 mAb. Cells were analyzed 4–16 h later for l-selectin expression by flow cytometry. Cell viability was unchanged after crosslinking of the CD4 receptor (data not shown). Fluorescence mean values of l-selectin expression on live Th cells are shown as percentage of the untreated control. P values and asterisks indicate significance and were obtained by applying the Wilcoxon signed rank test. (C) CFSE-labeled human CD4+ PBMCs were cocultured with HIV-1 NL4-3-infected (thick line) or mock-infected (thin line) Jurkat cells. Before coculture, Jurkat cells were incubated with medium, 10 μg/ml soluble CD4 or 10 μg/ml BSA. Cells were harvested after 12 h, and levels of l-selectin expression and CD69 expression on CFSE+ cells were determined by flow cytometry by using two-color staining. Results are representative of at least five experiments.