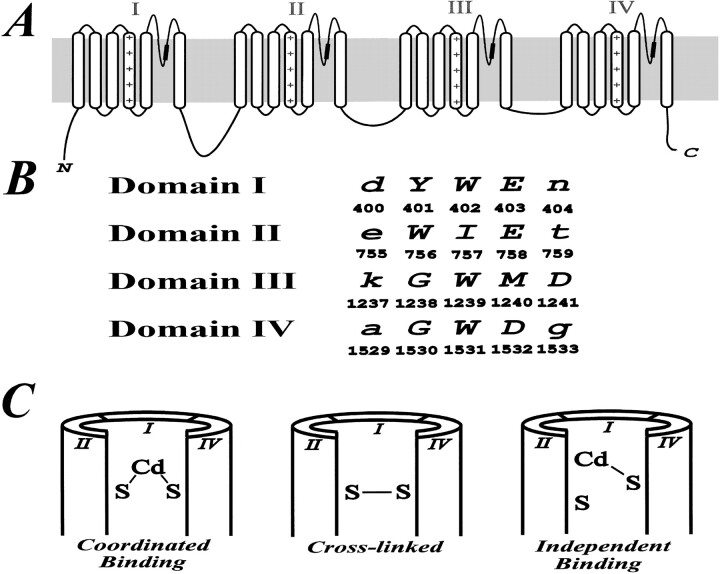
Figure 1.
(A) The putative topology of Na+ channels showing four homologous internal repeats each with six transmembrane segments (S1-S6). The four P-loops which are located between S5 and S6 in each repeat domain form a major part of the channel pore. The approximate location of residues mutated in our study are marked by a thickened portion of the COOH-terminal portion of the P-loop (i.e., SS2 domain). (B) Partial alignment sequences along with the corresponding residue numbers of the P-loops in the region which were mutated in our experiments. The mutated residues are marked as capital letters. (C) Three outcomes of introducing pairs of cysteine residues into distinct homologous repeat domains of Na+ channels are possible. Coordinated Cd2+ binding is expected if the two inserted sulfhydryls are sufficiently close to one another. Cross-linked channels are expected to be insensitive to current block by Cd2+ and will become more Cd2+-sensitive by reduction with DTT. If the two inserted cysteines are distant from one another, we expect Cd2+ to bind independently.