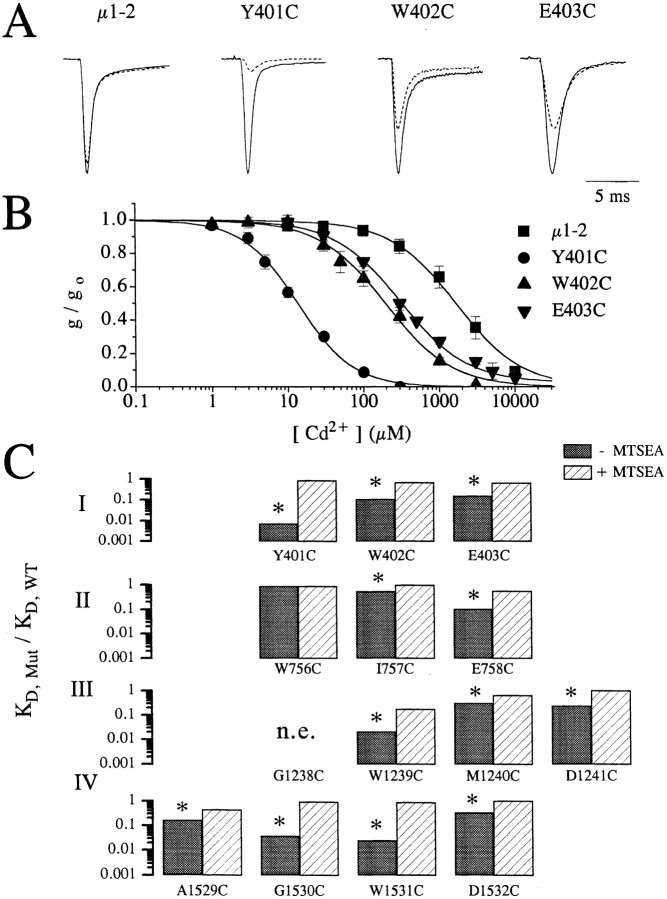
Figure 2.
(A) Raw current traces of wild-type, Y401C, W402C, and E403C channels recorded in oocytes following depolarization to −10 mV from a holding potential of −120 mV with (broken line) and without (solid line) 100 μM extracellular Cd2+. Clearly, the amount of current reduction is much greater for the mutant channels (Y401C, W402C, and E403C) than for the wild-type channels. The currents amplitudes have been scaled for ease of comparison. Peak currents in μA were: 3.8 (WT), 3.5 (Y401C), 3.5 (W402C), and 2.0 (E403C). (B) Plots of the fraction of peak current remaining as a function of the extracellular [Cd2+] for the same channels shown in A. Curve fits allow estimation of the dissociation constants (i.e., K D) for Cd2+ binding to the channel pore. (C) Summary of the Cd2+ block in single-cysteine mutants. The ratio of the estimated K D for Cd2+ block of current in single-cysteine mutants divided by the wild-type channels (i.e., K D,mut/K D,WT) for control conditions (filled bars) and following the application of 1.0 mM methane-thiosulfate-ethylammonium (MTSEA) to oxidize the inserted free sulfhydryls (open bars). For each mutant, except W756C, K D,mut was significantly reduced (p < 0.001) more than 3-fold, which was abolished by the application of MTSEA.