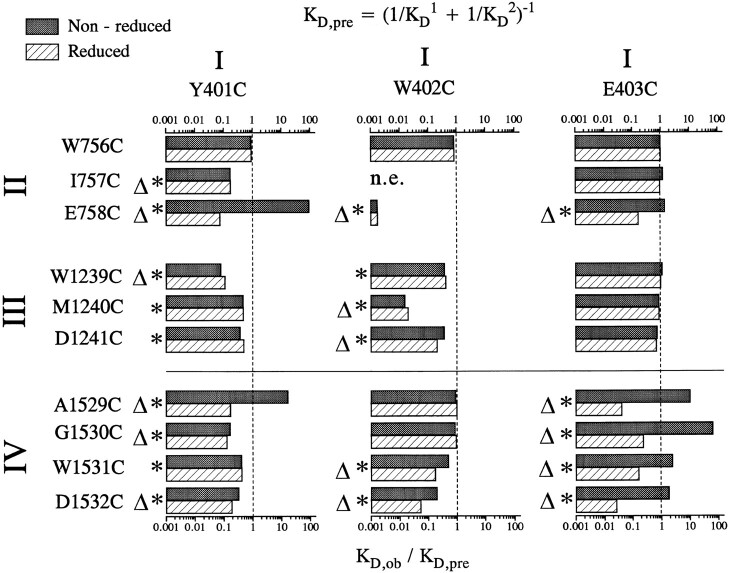
Figure 4.
Ratios of the predicted dissociation constant, K D,pre, to the experimentally observed dissociation constant, K D,ob for all the double mutants studied before (filled bars) and after (open bars) reduction by DTT. K D,pre is estimated from the dissociation constants recorded for the single-cysteine mutant channels assuming independent binding of Cd2+ to the inserted cysteines in double-cysteine mutants (see methods, Eq. 1). For the mutants with ratios of K D,ob/K D,pre around 1, we assume that the two inserted cysteines are binding Cd2+ independently. Values of K D,ob/K D,pre statistically different from 1 are identified by asterisks (*). The mutant channels with K D,ob/K D,pre below 0.37 (i.e., with stabilization energies of 1 kT) are identified by open triangles.