Abstract
Metabotropic γ-aminobutyric acid (GABA) receptors were studied in amphibian retinal ganglion cells using whole cell current and voltage clamp techniques. The aim was to identify the types of receptor present and their mechanisms of action and modulation. Previous results indicated that ganglion cells possess two ionotropic GABA receptors: GABAAR and GABACR. This study demonstrates that they also possess two types of metabotropic GABAB receptor: one sensitive to baclofen and another to cis-aminocrotonic acid (CACA). The effects of these selective agonists were blocked by GDP-β-S. Baclofen suppressed an ω-conotoxin–GVIA-sensitive barium current, and this action was reversed by prepulse facilitation, indicative of a direct G-protein pathway. The effect of baclofen was also partially occluded by agents that influence the protein kinase A (PKA) pathway. But the effect of PKA activation was unaffected by prepulse facilitation, indicating PKA acted through a parallel pathway. Calmodulin antagonists reduced the action of baclofen, whereas inhibitors of calmodulin phosphatase enhanced it. Antagonists of internal calcium release, such as heparin and ruthenium red, did not affect the baclofen response. Thus, the baclofen-sensitive receptor may respond to influx of calcium. The CACA-sensitive GABA receptor reduced current through dihydropyridine-sensitive channels. Sodium nitroprusside and 8-bromo-cGMP enhanced the action of CACA, indicating that a nitric oxide system can up-regulate this receptor pathway. CACA-sensitive and baclofen-sensitive GABAB receptors reduced spike activity in ganglion cells. Overall, retinal ganglion cells possess four types of GABA receptor, two ionotropic and two metabotropic. Each has a unique electrogenic profile, providing a wide range of neural integration at the final stage of retinal information processing.
Keywords: baclofen, cis -aminocrotonic acid, calcium/calmodulin, adenylate cyclase, guanylate cyclase
introduction
The retina has emerged as a model system for the study of γ-aminobutyric acid (GABA)1 receptors. It possesses a diversity of GABAA receptors (GABAAR) (Greferath et al., 1995), at least two types of GABAB receptor (GABABR) (Matthews et al., 1994; Tian and Slaughter, 1994), and a GABAC receptor (GABACR) (Cutting et al., 1991). These receptors produce a variety of electrogenic effects including the ionotropic increase in chloride conductance and the metabotropic modulation of a calcium conductance and activation of a putative potassium conductance. The ability of GABA to produce this multiplicity of effects permits a precise and varied integration of synaptic information.
While a plethora of GABA receptors have been identified in retina, often they have been discretely localized to small groups of neurons. For example, in white perch retina the GABACR has been described in a rod-driven horizontal cell (H4) (Qian and Dowling, 1993), while in catfish retina this receptor was found in cone-driven, but not rod-driven, horizontal cells (Dong et al., 1994; Takahashi et al., 1994). The baclofen-sensitive GABABR has been identified in fish ganglion cells but was not seen in fish horizontal or bipolar cells (Bindokas and Ishida, 1991). In one type of goldfish bipolar cell a cis -aminocrotonic acid (CACA)-sensitive, but not the baclofen-sensitive, GABA receptor was detected (Matthews et al., 1994). This restricted expression fits a model in which GABARs are precisely localized so that each receptor subtype is associated with particular neurons. The implication is that there is a functional correlation between receptor properties, such as conductance and kinetics, and the physiology of the neuron. In this context, Pan and Lipton (1995) reported that the GABACR found in rat bipolar cells produced a smaller and slower current than the GABAAR. It is tempting to conclude that there is a good match between this receptor and the relatively slow and small responses of the rod pathway.
An alternative model of GABA receptor diversity is that multiple GABARs may be localized in one cell and thereby expand the inhibitory capability of that neuron. If present, this confluence of GABA receptor subtypes could be particularly important in retinal ganglion cells, the recipients of powerful GABAergic input from amacrine cells and the final integrators of retinal information. Therefore, we examined retinal ganglion cells to determine their complement of GABA receptors. We previously reported that amphibian ganglion cells possess two types of ionotropic GABA receptor: GABAA and GABAC (Zhang and Slaughter, 1995). We find that these neurons also contain two types of metabotropic GABA receptor, one sensitive to baclofen and the other to CACA. Thus, ganglion cells possess every type of GABA receptor currently identified. Through these different receptors, GABA regulates chloride and potassium conductances and modulates voltage-dependent calcium conductances. This permits a graded level of inhibition by parallel deployment of different conductance mechanisms. The GABA receptors can be differentially modulated by the internal or external environment of the ganglion cell, indicating that the balance of receptor inhibition can be tuned.
materials and methods
Experiments were performed in aquatic tiger salamander (Ambystoma tigrinum) retinal slice preparation, which has been described in detail previously (Werblin, 1978; Wu, 1987). In brief, the animal was decapitated, double pithed, and the eye was enucleated. The cornea, lens, and vitreous were removed, exposing the retina. The retina was separated from the pigment epithelium and positioned, photoreceptor side down, on filter paper. The retina and filter paper were sectioned into 200–300-μm thick slices. These sections were placed on their sides in a perfusion chamber and positioned on the stage of an upright microscope fitted with Hoffman modulation optics. The retinal layers and individual neurons could be observed while a microelectrode was positioned for whole cell recording. Low resistance electrodes (<5 MΩ) were used to form gigaseals. Recordings were obtained from neurons in the ganglion cell layer, a somatic layer that Lukasiewicz and Werblin (1988) found to be almost exclusively populated by ganglion cells. In some cases the neurons were stained with Lucifer yellow (0.05% added to pipette solution) and retrospectively identified as ganglion cells.
A few experiments were performed on isolated ganglion cells. A similar dissection procedure and standard dissociation procedures were employed, as detailed previously (Pan and Slaughter, 1995).
Whole cell pipette solution consisted of (in mM): 106 K-gluconate, 5 NaCl, 2 MgCl2, 5 EGTA, and 5 HEPES, buffered to pH 7.4 with KOH. To reduce calcium channel run-down, the pipette solution also contained an “ATP-regenerating cocktail” consisting of 4 mM ATP, 20 mM phosphocreatine, and 50 U/ml creatine phosphokinase. In experiments to study the CACA-sensitive GABA receptor, the ATP-regenerating system was replaced with 4 mM ATP and 0.3 mM GTP. At the recommendation of one reviewer, a few experiments were performed using nystatin perforated patches. In these cases, the same internal solution was used without the ATP-regenerating medium.
Both voltage steps and ramps were used to evoke currents. Since the barium currents that we measured were sustained and showed very little inactivation over this time course, we found that results using the step and ramp were similar. For convenience, ramp data were most often obtained. The figures in this paper display the original data and are not corrected for electrode tip potential or series resistance. The cells had high input resistances so leak subtraction was not necessary. The tip potential was measured for each pipette solution (Neher, 1992), and the correction factor is mentioned in results, particularly where reversal potential measurements are presented. The amplifier did not have series resistance compensation. Except for perforated patch recordings, access resistance was generally between 6 and 15 MΩ. This resulted in an offset in the peak barium currents. However, since we were monitoring sustained currents and examining these currents over a wide range of potentials, this offset did not significantly influence our results. In a few cases, where a large shift occurred, the data were discarded. When both tip potential and series resistance voltage offsets were subtracted, the barium current was calculated to peak near +10 mV. This information was not a topic of this paper, so these calculations were not routinely done for each cell and the raw data is shown without these corrections. Voltage clamp experiments were performed using an Axoclamp 2A amplifier in continuous voltage clamp mode and PCLAMP software on a Gateway 486 computer equipped with a Labmaster A/D board. Analysis was performed employing PCLAMP, EXCEL, and Origin software.
Control amphibian Ringer's solution consisted of (in mM): 111 NaCl, 2.5 KCl, 1.8 CaCl2, 1 MgCl2, 10 dextrose, and HEPES buffered to pH 7.8 and oxygenated. When calcium currents were studied in isolation, 10 mM BaCl2 and 40 mM tetraethyl ammonium chloride (TEA·Cl) replaced equimolar NaCl in the extracellular medium. Tetrodotoxin (1 μM) was used to block sodium currents. Application of 25 μM CdCl2 suppressed most of the inward current under these conditions, indicating that most, if not all, of the observed current arose from calcium channels. Control and drug containing solutions were applied through a gravity fed system to a manifold in connection with the perfusion chamber containing the retinal slice. Valves controlled drug application, and there was a delay of ∼10 s due to exchange time of the system. Alternatively, drugs were applied through a puff pipette positioned at the edge of the retina near the cell of interest. In this case, Ringer's solution was still constantly applied to the retinal slice. When antagonists were tested, they were applied first, then after 30–60 s agonist was applied with antagonist. Generally, one cell was recorded in each retinal slice to avoid residual drug effects. Drug effects described in this paper are steady-state values, usually obtained 30–60 s after drug application.
The drug concentrations required to evoke effects in the slice preparation were often higher than anticipated, indicating that there was a diffusion or uptake barrier. For example, 100 μM GABA had only a small effect in the slice preparation, although it produced a near maximal effect when applied to isolated ganglion cells (Pan and Slaughter, 1995). This was true for glutamate as well (Shen and Slaughter, unpublished observations). Therefore, there is probably a disparity between the dose applied and the effective drug concentration at the receptor. This phenomenon has been noted in retinal slice studies from other laboratories. Also, during the course of these studies we noted a decrease in the response to baclofen and to cis -aminocrotonic acid. Whether this represented a seasonal change or other effect was unclear. A series of initial experiments were performed using 100 μM baclofen, later 50 μM baclofen was used. In results, the average effect of 100 μM baclofen is reported to be greater than the effect of 50 μM, but this is probably due to the time periods when the studies were completed. Some experiments compare the effects of second messenger antagonists with the average effect of 100 or 50 μM baclofen. These antagonist experiments were performed in periods that overlapped the experiments from which the average data were obtained, and therefore represent ganglion cell populations with appropriate control responses. In CACA experiments, 5 μM doses produced nearly 50% suppression in early experiments (winter), but only about 20% suppression in later experiments (summer). Again, this may have been due to seasonal changes or other factors. When comparing data between experiments, we only contrasted effects measured at the same time period (usually the same day or week). All statistical data are presented as means ± SEMs.
SR95531, 2-hydroxysaclofen, nifedipine, nimodipine, trifluoperazine, KN-62, forskolin, H-9, Rp-cAMP, microcystin, calmidazolium, ruthenium red, heparin, NG-monomethyl-l-arginine, and sodium nitroprusside were purchased from Research Biochemicals, Inc. (Natick, MA). CACA was obtained from Tocris Cookson (St. Louis, MO). Cyclosporin A was obtained from Calbiochem Corp. (San Diego, CA). CGP35348 and baclofen were gifts from Ciba-Geigy (Basel, Switzerland). All other chemicals were obtained from Sigma Chemical Co. (St. Louis, MO).
results
GABA-regulated Currents in Ganglion Cells
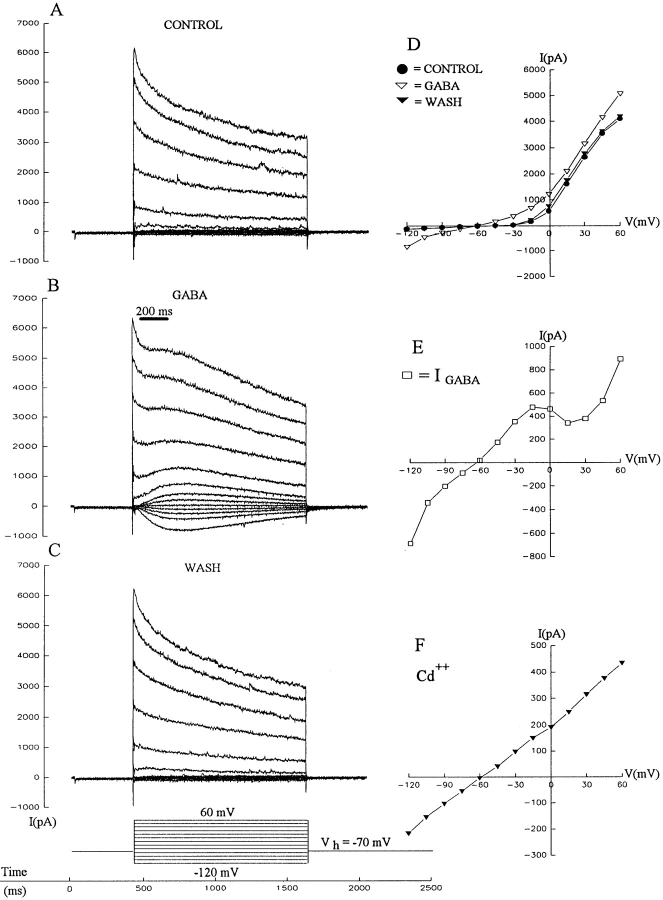
The effect of GABA was tested on ganglion cells in the retinal slice preparation by holding neurons at −70 mV and then stepping them to various potentials between −120 and +60 mV, as illustrated in the left column of Fig. 1. Under control conditions (Fig. 1 A), ganglion cells exhibited two prominent currents: a fast inward sodium current (that was truncated in this figure) and a large outward potassium current. The effects of a localized puff of GABA (Fig. 1 B) and recovery (Fig. 1 C) are shown, and the I-V relationships under all three conditions are illustrated (Fig. 1 D). The currents were measured at a time corresponding to the peak of the GABA induced current in Fig. 1 B. The difference current (drug minus control), representing the isolated GABA current, is plotted in Fig. 1 E. This figure shows that the GABA-induced current is fairly linear between −100 and −20 mV, and reverses near −60 mV. Correcting for the tip potential indicates that this current reverses at −69 mV, close to the estimated chloride reversal potential (−72 mV). Numerous reports indicate that a large portion of this chloride current is due to activation of GABAARs (Miller et al., 1981; Ishida and Cohen, 1988; Cohen et al., 1989; Müller et al., 1992; Lukasiewicz and Werblin, 1994). We found that GABA-activated chloride current in salamander ganglion cells results from activation of both GABAA and GABAC receptors (Zhang and Slaughter, 1995).
Figure 1.
GABA induced currents in retinal ganglion cells. This shows the current responses of a ganglion cell to voltage steps under control conditions (A), during 200-ms puffs of 500 μM GABA from a pipette near the proximal edge of the retina (B), and after cessation of GABA application (C). The current at the end of the GABA puff was plotted for each voltage under all three conditions (D), and the GABA current was calculated by subtracting the control currents from the GABA currents, and this is plotted (E). When the calcium current was blocked by 100 μM cadmium, then GABA puffs produced currents that did not show a notch in the I-V curve (F). Recordings A–E were obtained from the same cell; results shown in F were from another neuron. Recordings were obtained from ganglion cells in the retinal slice preparation superfused with normal Ringer's solution. Cells were clamped to −70 mV and then stepped to voltages ranging from −120 mV to +60 mV in 15-mV increments as illustrated. Symbols in panels E–F indicate the currents observed at each voltage step, the line connecting these data points was drawn by eye.
A notch is apparent in the I-V curve (Fig. 1 E) in the region where high voltage–activated (HVA) calcium currents might arise. This suggests that an additional effect of GABA is to suppress a voltage-activated calcium conductance, resulting in a reduction in an outward potassium conductance. The calcium current was blocked to test this hypothesis. When a protocol similar to that in Fig. 1 E was used in the presence of 100 μM external cadmium (this recording is from a different neuron than the one in Fig. 1, A–E), the GABA I-V curve was relatively linear and lacked the notch (Fig. 1 F). The data support the supposition that the notch was due to the GABA-induced reduction of a calcium-activated outward current. Ensuing experiments indicate that GABA does suppress calcium channel currents.
Receptor Modulation of Calcium Currents
In a number of systems, a baclofen-sensitive GABABR has been associated with the down-regulation of calcium currents. In retina, this has been reported in a subset of amphibian bipolar cells (Maguire et al., 1989) and in goldfish ganglion cells (Bindokas and Ishida, 1991). We therefore examined the effect of baclofen on calcium channel current in amphibian ganglion cells. The measurement of calcium channel current required a modified Ringer's solution, which contained 10 mM barium, 40 mM TEA-Cl, and was nominally calcium free. Under these conditions we observed an HVA, relatively sustained inward current that was blocked by 100 μM external cadmium (data not shown). In all these experiments, barium was the charge carrier. Since this current was relatively noninactivating, we found that similar data were obtained using either voltage steps or ramps.
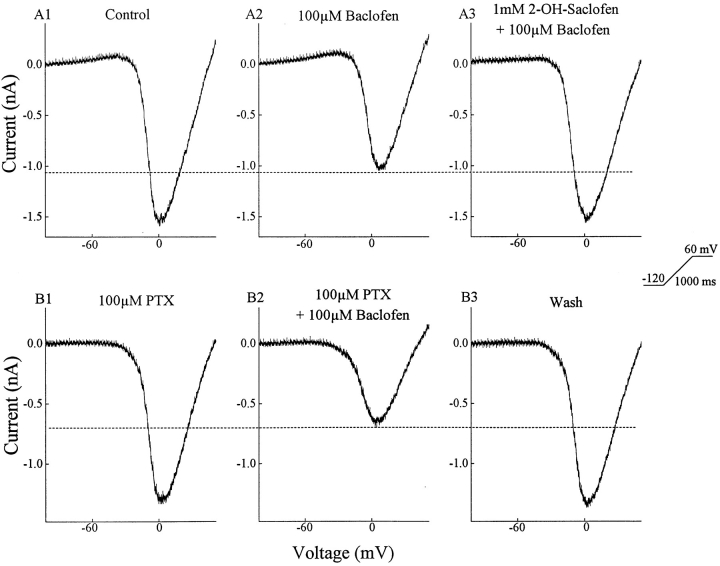
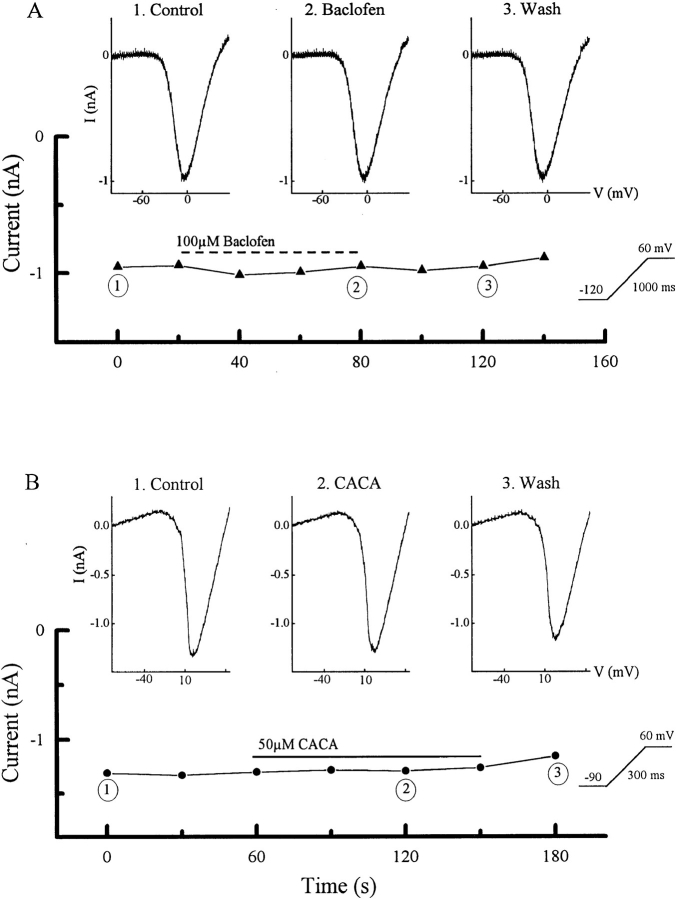
Baclofen suppressed a portion of the HVA barium current in ganglion cells (Fig. 2). The neuron was held at −70 mV, stepped to −120 mV, and then the voltage was ramped to +60 mV in 1 s. This paradigm evoked an inward barium current (Fig. 2 A1) that was suppressed approximately 30% by 100 μM baclofen (Fig. 2 A2). Adding 2-hydroxysaclofen (1 mM), an antagonist of baclofen-sensitive GABA receptors (Kerr et al., 1988), restored the inward current close to its control value (Fig. 2 A3). The lower three panels, taken from recordings of another ganglion cell, show that picrotoxin, an antagonist of both GABAARs and GABACRs, did not block the effect of baclofen on the HVA barium current. In experiments on 73 cells, 50 μM baclofen reduced the HVA current by 29 ± 1% (mean ± SEM), while in another 22 ganglion cells 100 μM baclofen reduced the barium current by 40 ± 3% (see materials and methods regarding this difference). In the presence of 100 μM picrotoxin, 100 μM baclofen reduced the barium current by 44 ± 3% (n = 6), indicating that picrotoxin did not reduce baclofen's action. This pharmacology leads to the conclusion that amphibian ganglion cells possess a baclofen-sensitive GABABR that can down-regulate HVA calcium currents.
Figure 2.
Baclofen reduces a voltage-activated barium current. The voltage was ramped from −120 to +60 mV in 1 s. The barium current was isolated and enhanced by replacing extracellular calcium with 10 mM barium and adding 40 mM TEA (equimolar replacement of NaCl). The top three panels show that 100 μM baclofen reduced the barium current, and the effect of baclofen was reversed by 1 mM 2-hydroxysaclofen. The lower set of panels confirms that 100 μM picrotoxin did not block the effect of baclofen.
In previous studies (Tian and Slaughter, 1994), CGP35348 was found to block baclofen but 2-hydroxysaclofen did not. In the present experiments, both were effective baclofen antagonists. The difference is that in the prior experiments the concentration of 2-hydroxysaclofen was 100 μM while in the present experiments that concentration was raised to 1 mM.
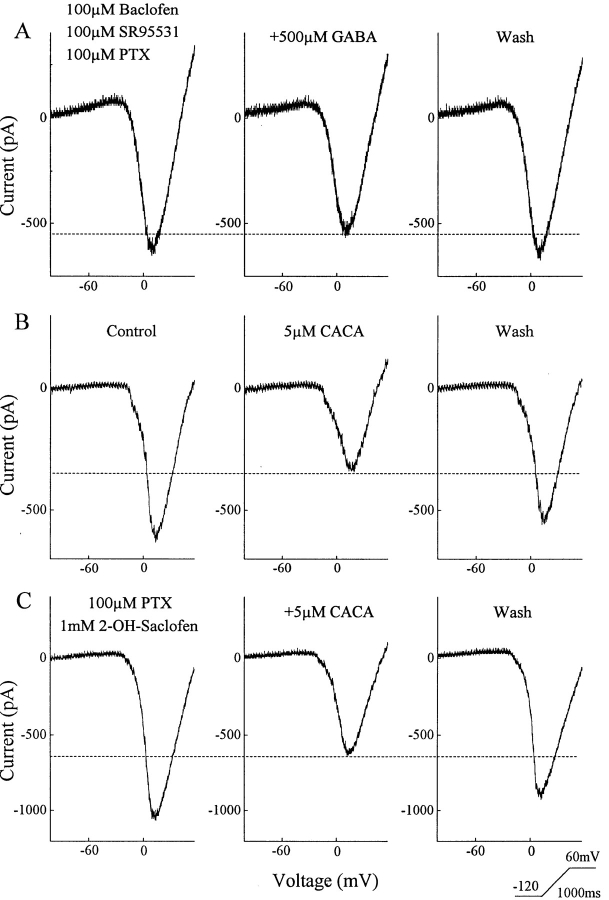
To determine if ganglion cells possessed another GABA receptor, we applied GABA in the presence of antagonists of the GABAAR, GABACR, and the baclofen-sensitive GABABR. Since 2-hydroxysaclofen is a weak and competitive antagonist of baclofen-sensitive GABABRs, we sometimes chose to saturate the baclofen-sensitive receptor instead of blocking it. When GABA was applied in the presence of SR95531, picrotoxin, and baclofen, it was still able to produce an additional suppression of the barium current (Fig. 3 A). In nine cells tested in this manner, GABA reduced the barium current by 11 ± 2%. Another GABAR has been identified in goldfish retina and shown to down-regulate HVA calcium channels in bipolar cells (Matthews et al., 1994). This has been proposed as a novel type of GABABR. It can be identified pharmacologically by its sensitivity to low micromolar concentrations of CACA and its insensitivity to bicuculline, SR95531, picrotoxin, baclofen, or 2-hydroxysaclofen.
Figure 3.
Ganglion cells possess a CACA-sensitive GABA receptor. (A) There is a GABA effect on barium current not due to the GABAAR, the baclofen sensitive GABABR, or the GABACR. When these receptors were blocked (GABAAR and GABACR) or saturated (GABABR), GABA still caused a reversible reduction in the voltage activated barium current. (B) Low doses of CACA reduced the barium current. (C) This effect of CACA was not blocked by a combination of antagonists that together block the GABAAR, GABACR, and the baclofen-sensitive GABABR.
This receptor is also present in amphibian ganglion cells. A low micromolar concentration of CACA was able to reduce the HVA barium current in a reversible manner (Fig. 3 B). CACA produced a 40% decrease in the barium current in this neuron. We saw no evidence that low doses of CACA changed the conductance of the cell (monitored at negative potentials where voltage-dependent channels were not activated), although at much higher concentrations it can activate the GABAAR as well as the GABACR in these cells (Zhang and Slaughter, 1995). The lower set of panels, taken from another ganglion cell, show that the effect of CACA persisted when it is applied in the presence of picrotoxin, which blocked the GABAA&CRs, and 2-hydroxysaclofen, which blocked the baclofen-sensitive GABABR. As noted by Matthews et al. (1994), CACA effects were robust when the inside of the cell was dialyzed with GTP. With 0.3 mM GTP in the pipette, CACA reduced the barium current by a mean of 46 ± 5% in nine cells. When GTP was not included in the pipette solution, CACA produced a smaller reduction in the barium current of 23 ± 2% in 14 cells. This is different than the action of baclofen, which did not depend on GTP dialysis. Without GTP dialysis the recovery after CACA treatment was poor. All subsequent CACA experiments described in this report were performed using GTP dialysis. The GABA experiments in Fig. 3 did not include GTP.
Since different internal solutions were used in recording baclofen-sensitive and CACA-sensitive currents, nystatin perforated patch recordings were used to compare the actions of baclofen, CACA, and GABA while limiting the effects of cell dialysis. High concentrations were used to produce maximal responses to each agonist (50 μM CACA, 100 μM baclofen, and 200 μM GABA). The retina slice was pretreated and continuously superfused with picrotoxin (200 μM) to avoid the effects on ionotropic GABA receptors. In five cells, baclofen was found to reduce the barium current by a mean of 28% (from a control value of 1,028 ± 169 pA to 708 ± 109 pA); CACA reduced the current by 9% (to 924 ± 135 pA); GABA reduced the current by 46% (to 534 ± 82 pA). The response to each agonist was statistically significant (P < 0.05, Wilcoxin's signed-ranks test).
The ionotropic GABACR is sometimes referred to as CACA-sensitive because CACA can be more effective at activating the GABACR than the GABAAR (Feigenspan et al., 1993; Qian and Dowling, 1993; Pan and Lipton, 1995). We previously reported that high concentrations of CACA were needed to stimulate the GABACR, and these concentrations also activated the GABAAR (Zhang and Slaughter, 1995). This is in contrast to the receptor identified in Fig. 3, which is sensitive to low micromolar concentrations of CACA. To avoid confusion with reports describing CACA sensitivity of the GABACR or GABAAR, we refer to this receptor as the CACA-sensitive GABABR (GABAB-CACAR).
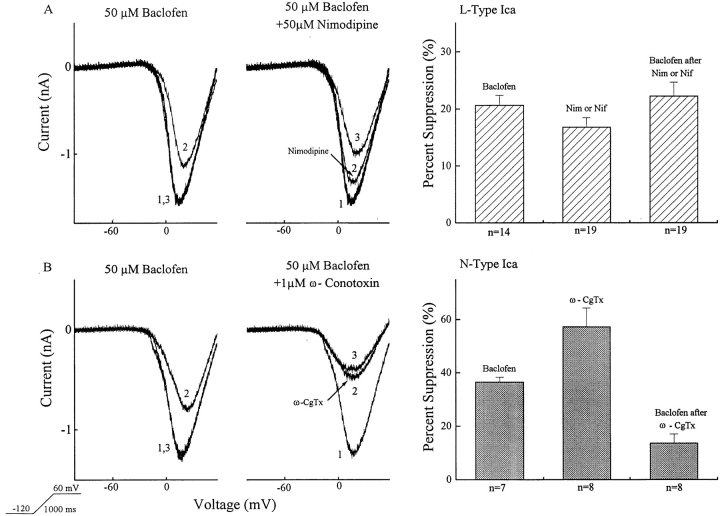
Baclofen's Action on Different Types of Calcium Channels
Calcium channel blockers were employed to evaluate the characteristics of the currents regulated by baclofen or CACA. The left side of Fig. 4 shows examples of ramp-elicited barium currents in the presence of baclofen alone, the blocker alone, and the combination of baclofen with the channel blocker. 19 ganglion cells were used to test the effects of two L-type calcium channel blockers: nimodipine and nifedipine. The effect of 50 μM baclofen alone was tested in 14 of the 19 cells and found to reduce the barium current by 21 ± 2%. In the 19 cells, 50 μM nifedipine or nimodipine was applied. This is a concentration found to block baclofen's effect on L-type calcium channels in bipolar cells in the same preparation (Maguire et al., 1989). These dihydropyridines reduced the barium current by 17 ± 2%. In the presence of nifedipine or nimodipine, baclofen reduced the calcium current in these 19 cells by an additional 22 ± 2%. Thus, baclofen produced approximately the same percent suppression of the calcium current whether the dihydropyridine channel blockers were present or not. If baclofen had no effect on the L-type calcium current, then the percentage suppression by baclofen should be greater in the presence of these blockers. That is, if these two effects were independent they should be additive. On average, the percent suppression by baclofen was slightly greater in the presence of the blockers, but this was not statistically significant. Since the blockers reduced the calcium current by 17% on average, an additive effect would only alter the percent suppression by baclofen from a mean suppression of 21% to a mean suppression of 25% (compared to the observed mean suppression of 22%). Therefore, we can conclude that baclofen does not act primarily on L-type channels, although we cannot exclude a small modulation of L-type calcium channels.
Figure 4.
The GABAB-BACLOFENR suppresses a ω-conotoxin–GVIA-sensitive calcium channel. The upper left panel in A shows that baclofen reversibly reduces barium current (1 = control; 2 = baclofen; 3 = recovery). The adjacent panel in A, from the same cell, illustrates that 50 μM nimodipine (2) reduces the control barium current (1), but 50 μM baclofen in the presence of nimodipine (3) further reduces the current. A summary of the results is charted in the upper right panel. In total 19 neurons were tested, but in only 14 of the 19 was baclofen alone tested. The bottom row illustrates similar studies performed using ω-con-otoxin-GVIA (ω-CgTx) (2), an N-type calcium channel blocker, followed by application of 50 μM baclofen in the presence of ω-CgTx (3). A summary of data on eight neurons appears in lower right panel. Seven of the eight were tested with baclofen alone.
The N-type channel blocker, ω-conotoxin–GVIA, had a much larger effect on both the calcium current and baclofen's action (Fig. 4, bottom). In eight cells, ω-conotoxin reduced the barium current by 57 ± 7%. In the presence of ω-conotoxin, 50 μM baclofen produced only a 14 ± 3% suppression of the remaining reduced barium current. Of the eight cells used in these experiments, seven had been previously tested with baclofen alone. Baclofen had produced a mean barium current suppression of 37 ± 2%. If baclofen acted exclusively on non–N-type calcium channels, it would be expected to suppress 86% of the remaining calcium current in the presence of ω-conotoxin. If ω-conotoxin-GVIA is acting selectively, this indicates that a large fraction of the baclofen effect is on the N-type channel.
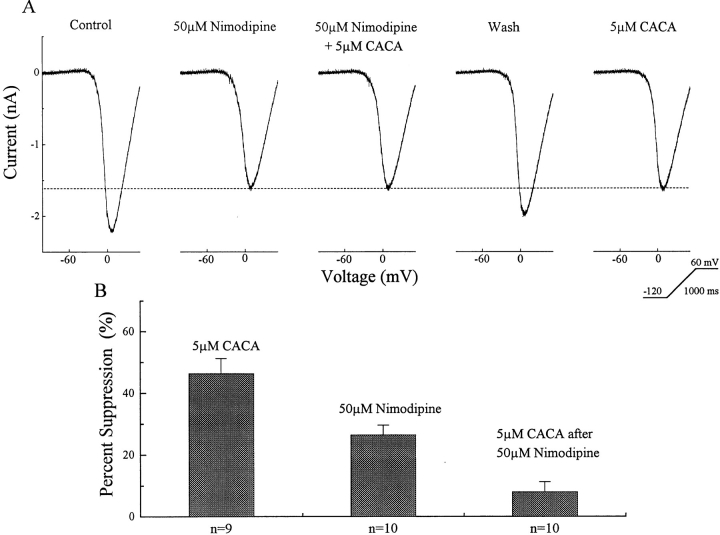
The CACA-sensitive GABAB Receptors Act on L-type Calcium Channels
In contrast to the effect of the GABAB-BACLOFENR, the GABAB-CACAR appears to act predominantly at the dihydropyridine-sensitive calcium channel (Fig. 5). The top row in this figure shows a recording from one ganglion cell in which 50 μM nimodipine reduced the voltage-activated barium current. In the presence of nimodipine, 5 μM CACA had little additional effect. After washout and recovery of the barium current, CACA alone was able to reduce the barium current. The reduction by CACA was similar to that produced by nimodipine. A summary of data is charted in the lower portion of Fig. 5. The CACA alone data in this chart are drawn from our initial study of nine ganglion cells in which 5 μM CACA suppressed a mean of 46 ± 5% of the barium current. In a different set of ten ganglion cells, 50 μM nimodipine reduced the barium current by 25 ± 3%. In these ten neurons, after nimodipine reduced the barium current, CACA in the presence of nimodipine produced an additional barium current reduction of 9 ± 3%. All recordings were performed with 0.3 mM GTP in the patch electrode.
Figure 5.
The GABAB-CACAR modulates a dihydropyridine-sensitive barium current. The top trace shows recordings from one ganglion cell in which the barium current was evoked by a voltage ramp. The current was suppressed by 50 μM nimodipine or 5 μM CACA. In the presence of nimodipine, the effect of CACA was occluded. The chart shows data on a population of nine ganglion cells in which CACA suppressed the barium current and another set of ten ganglion cells in which nimodipine reduced the barium current and largely occluded the action of CACA.
GABABR Effects on Ganglion Cell Spike Activity
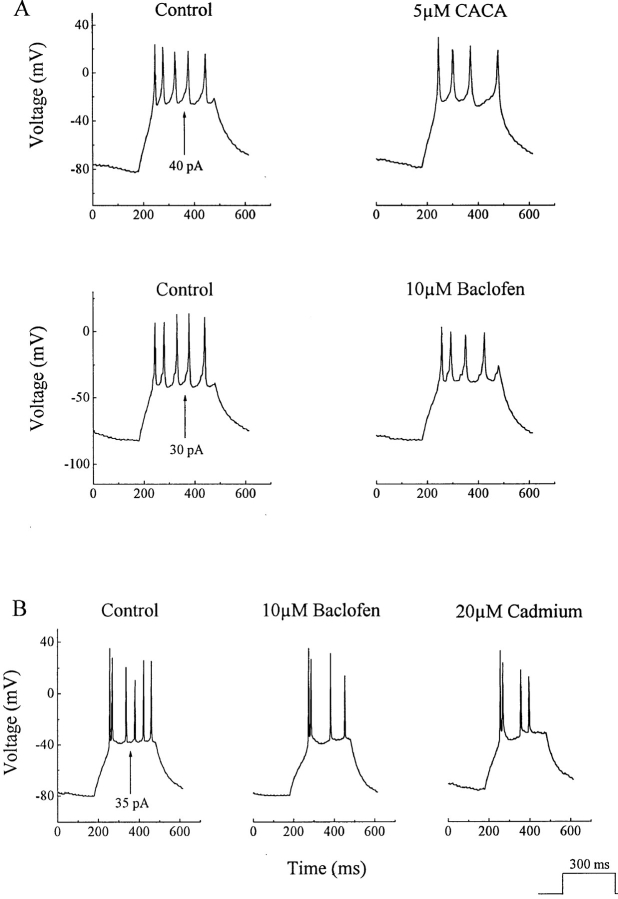
A simple method of analyzing the influence of metabotropic GABA receptors on ganglion cells is to use current clamp recordings. The protocol for these experiments was to record from neurons in the ganglion cell layer under whole cell current clamp and evoke spike activity by applying 300-ms positive current pulses under control conditions and during application of GABA agonists (see Fig. 7 A). Both baclofen and CACA produced modest reductions in ganglion cell spike activity (Fig. 6 A). In experiments on 16 cells, baclofen reduced the number of spikes in every case, with a mean reduction of 28 ± 12% (P < 0.01, Wilcoxin's signed-ranks test). CACA reduced spike number in all twelve cells tested, with a mean reduction of 22 ± 8% (P < 0.01, Wilcoxin's signed-ranks test). We also noted that both agonists produced spike broadening of approximately 30%. Calcium-activated potassium channels alter spike frequency (Hille, 1992), which probably explains these GABAB receptor actions. This assumption is supported by the observation that cadmium, a calcium channel antagonist, produced effects that were very similar to CACA or baclofen. An example is shown in Fig. 6 B, which compares the effects of cadmium and baclofen on responses to current injections in a ganglion cell. Liu and Lasater (1994a) found that dopamine produced a similar, though more powerful, suppression of spike activity in turtle ganglion cells.
Figure 7.
GDP-β-S blocks the effects of CACA and baclofen. Whole cell recordings were performed while including 1 mM GDP-β-S in the pipette solution. The graphs show the peak barium current measured at fixed intervals before, during, and after drug application.
Figure 6.
Effects of CACA and baclofen on ganglion cell spike activity. (A) Ganglion cell spiking was elicited by brief (300 ms) current injections. Both baclofen and CACA decreased spike activity. (B) The reduction in spike activity produced by baclofen was duplicated by cadmium.
Baclofen and CACA Act through G-protein Pathways
Transmitter-receptor systems that modulate voltage- activated calcium channels are generally metabotropic and blocked by agents that interfere with the G-protein cascade. This was tested using guanosine 5′-O-(2-thiodiphosphate) (GDP-β-S), which blocks G-protein–mediated pathways (Fig. 7). The protocol was similar to previous experiments except that 1 mM GDP-β-S was included in the pipette. A calcium current was periodically evoked, then baclofen or CACA was applied. The graphs show the peak currents before, during, and after prolonged drug application. Baclofen and CACA produced only a slight reduction in the current. Representative currents, measured at the points marked by numbers on the graphs, are shown. Similar results were obtained in six other ganglion cells, indicating that both CACA and baclofen required functional G-protein systems.
Baclofen's Second Messenger Pathway
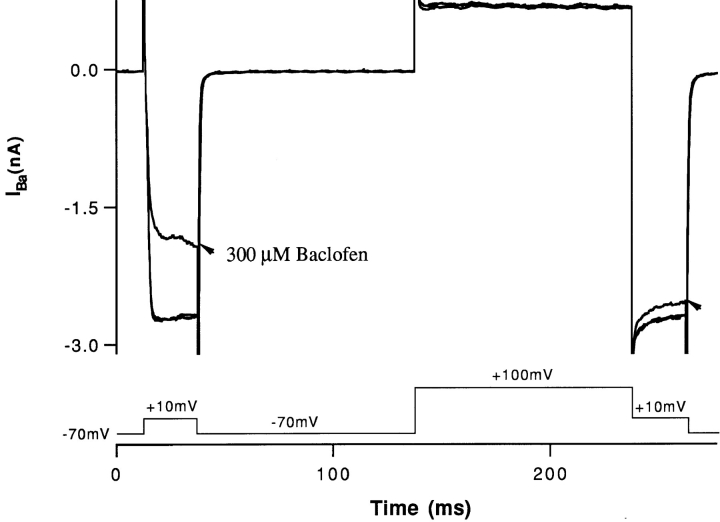
The effect of baclofen was reversed by prepulse facilitation. These experiments were performed in isolated cells. The protocol was to activate high voltage–activated calcium channels by depolarizing the cell to +10 mV, from a holding potential of −70 mV. Then the cell was prepulsed to +100 mV, then again clamped to +10 mV. Fig. 8 shows superimposed currents obtained under control conditions and in the presence of baclofen. High concentrations of baclofen were required to activate the GABA receptor in the isolated cell preparation. The reason for this is currently being investigated. However, the effects of baclofen in isolated cells were similar to those found in the slice, both in terms of pharmacology (2-hydroxysaclofen sensitivity) and electrogenic action. Under control conditions, the barium current was of similar magnitude before and after the prepulse. But, in the presence of baclofen, the barium current was much larger after the prepulse (arrows). The peak of the barium current in baclofen, after the prepulse, was similar to the magnitude of the barium current before baclofen application. Prepulse facilitation has been reported for a number of metabotropic transmitter systems, including the GABABR. It has been suggested the phenomenon is indicative of a direct G-protein modulation of the calcium channel (Boland and Bean, 1993; Campbell et al., 1995).
Figure 8.
Depolarizing prepulses reduce the effect of baclofen. Barium currents were elicited by stimulating pulses of +10 mV. If the conditioning potential was −70 mV, baclofen significantly reduced the barium current. But after a conditioning potential of +100 mV, baclofen had much less effect on the barium current evoked by the +10 mV pulse.
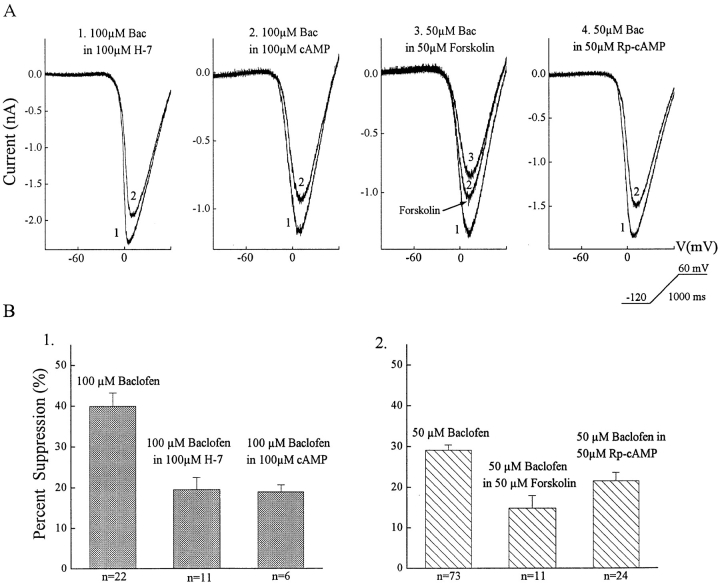
The effect of baclofen was reduced, but not eliminated, by a variety of agents that interfere with the protein kinase A (PKA) pathway. Examples of the effects of these agents and a summary of the data are shown in Fig. 9. Except for forskolin, all drugs were dialyzed into the cell by including them in the pipette solution. Therefore, the effects of baclofen during dialysis of these drugs were compared to effects of baclofen on a control population of neurons. The control data used, presented above, are that 50 μM baclofen reduced the HVA calcium current by a mean of 29% (n = 73), whereas 100 μM baclofen reduced this current by 40% (n = 22). When H-7, a protein kinase inhibitor, was dialyzed into 11 cells, the effect of 100 μM baclofen was reduced to approximately half the normal magnitude (19 ± 3%). But Rp-cAMP, a specific blocker of protein kinase A, had little effect on the response to baclofen. In the presence of Rp-cAMP, 50 μM baclofen suppressed the calcium current by 22 ± 2% (n = 24), about 75% of control. If the internal levels of cAMP were increased by dialysis then the effect of 100 μM baclofen was reduced by about 50% (19 ± 2% suppression in six cells). Forskolin was superfused onto the retina, and, therefore, the effect of forskolin on the calcium current could be measured. When ganglion cells were exposed to forskolin, it produced a 27 ± 3% suppression of the calcium current. And in the presence of forskolin, 50 μM baclofen produced a 15 ± 3% reduction in the calcium current (n = 11). If baclofen was acting on calcium channels that were not affected by forskolin, a suppression of 44% by baclofen would be anticipated. Therefore, the action of baclofen was significantly occluded by forskolin. The results suggest that activation of the PKA pathway interferes with the effects of GABAB-BACLOFENR stimulation.
Figure 9.
PKA analogues reduce the effect of baclofen. The top row (A) shows examples for each of the four agents tested while the bottom row (B) graphs the data for all cells tested. In the top row, trace 1 = PKA analogue and trace 2 = baclofen + PKA analogue except for the forskolin figure, where trace 1 = control, trace 2 = forskolin, and trace 3 = baclofen plus forskolin.
A few antagonists of other second messenger kinase pathways were tested and found not to alter the action of baclofen. These included a protein kinase C inhibitor, staurosporine, and a tyrosine kinase inhibitor, genistein.
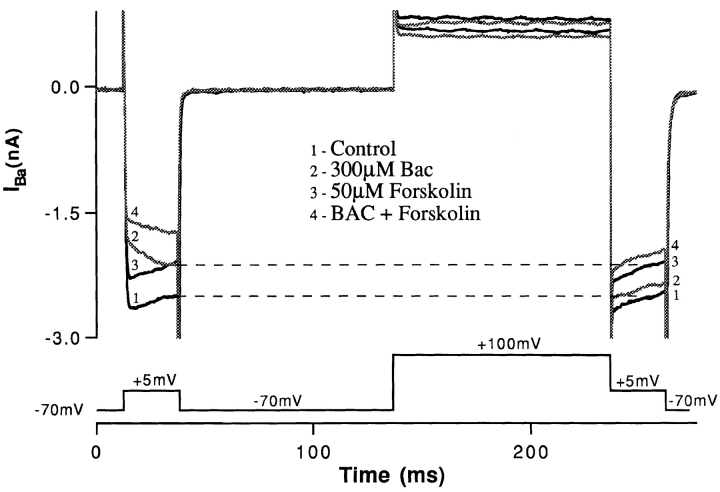
The effects of PKA agonists and antagonists suggest a role for this pathway in mediating the response to baclofen, whereas the prepulse data suggest a direct G-protein action that is independent of second messengers such as PKA. This discrepancy could be explained if the PKA system autonomously regulated the baclofen-sensitive calcium channel. Prepulse experiments supported this proposition (Fig. 10). While the effect of baclofen was reversed by prepulse facilitation (response 2 in Fig. 10), the action of forskolin was unaffected by the prepulse (response 3). In addition, the effect of baclofen and forskolin together (response 4) was less than the sum of each agent individually. This indicates that baclofen and forskolin act in parallel to reduce the calcium channel activation. Forskolin acts by a mechanism that differs from that produced by GABAB-BACLOFENR activation, yet occludes the effect of baclofen.
Figure 10.
Prepulse facilitation does not influence the effect of PKA. The left side of this figure shows that when barium currents are elicited by stepping to +5 mV from a holding potential of −70 mV (1), baclofen reduces more barium current (2) than does forskolin (3). But after a conditioning pulse to +100 mV, the effect of forskolin is the same but that of baclofen is suppressed and is now less than that of forskolin.
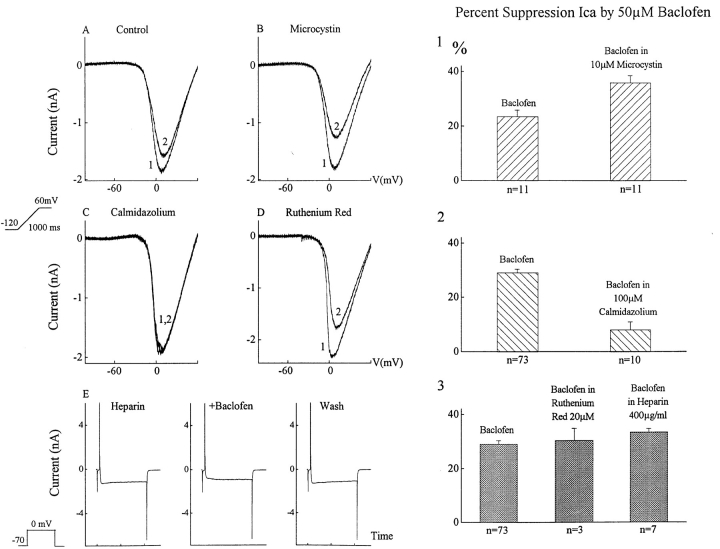
The action of baclofen was suppressed by calmidazolium, an inhibitor of calcium-calmodulin. In ten ganglion cells, internal dialysis with 100 μM calmidazolium reduced the effect of baclofen by an average of 72% (Fig. 11 C). Internal dialysis with 100 μM trifluoperazine, another calcium-calmodulin inhibitor, reduced baclofen's action by 57% (n = 7). A third inhibitor, W-7 (100 μM added to pipette), suppressed 43% of the baclofen effect (n = 5).
Figure 11.
Calmodulin-dependent enzymes regulate the GABAB-BACLOFENR. This figure shows the effects of baclofen on barium currents in the presence of agents that inhibit calcium-calmodulin (Calmidazolium), phosphatases (Microcystin), and internal calcium release (Heparin and Ruthenium Red). (A) Baclofen (2) reduces the barium current. (B) Microcystin alone has little effect on this barium current (1) (recording from the same cell as in A), but the effect of baclofen was enhanced by microcystin (2). (C) Calmidazolium (1) (added to whole cell pipette) suppressed the effect of baclofen (2), while heparin and ruthenium red (both added to whole cell pipette) did not reduce the effect of baclofen. The left side of this figure contains examples from single ganglion cells while the right side of the figure charts the effects on the population of neurons examined.
Calcium-calmodulin can activate protein kinases and phosphatases. We therefore examined the effect of microcystin LR, an inhibitor of protein phosphatases. An example is shown in Fig. 11, where 50 μM baclofen (Fig. 11 A, trace 2) suppressed the control barium current (trace 1) by 14% in this cell. Microcystin alone produced a very small suppression of the barium current in the same cell (Fig. 11 B, trace 1). But 50 μM baclofen in the presence of microcystin (trace 2) reduced the barium current by 29%. In eleven cells tested in this manner, microcystin enhanced the effect of 50 μM baclofen by 161% (from a mean calcium current suppression of 23 ± 3% to a mean of 37 ± 3%). Internal dialysis of cyclosporin A, a calcineurin phosphatase inhibitor, augmented baclofen's mean effect to 152% of control (n = 7). Okadaic acid, another phosphatase inhibitor, produced a similar enhancement. While blockers of calmodulin-dependent phosphatases had a dramatic effect on baclofen's action, KN-62, a very potent blocker of some calcium-calmodulin kinases, had only a slight suppressive effect (data not shown). Internal dialysis with 50–100 μM KN-62 reduced the baclofen effect by 22% (n = 5).
We did not find any evidence that the calcium-calmodulin modulation of the GABABR pathway resulted from calcium release from internal stores. Neither heparin, a blocker of the IP3 pathway, nor ruthenium red, a blocker of the ryanodine receptor, reduced the effect of baclofen (Fig. 11, D and E). Ruthenium red was tested by placing it in the electrode because extracellular application significantly reduced membrane barium currents. In three cells, 50 μM baclofen in the presence of ruthenium red reduced the barium current by 30 ± 5%. Heparin was also included in the patch pipette solution. After heparin dialysis, 50 μM baclofen still reduced the barium current by 33 ± 1% (n = 7). This suppression by 50 μM baclofen, in the presence of ruthenium red or heparin is very similar to the average effect of 50 μM baclofen alone (29 ± 1% suppression).
Modulation of CACA-sensitive GABAB Receptors
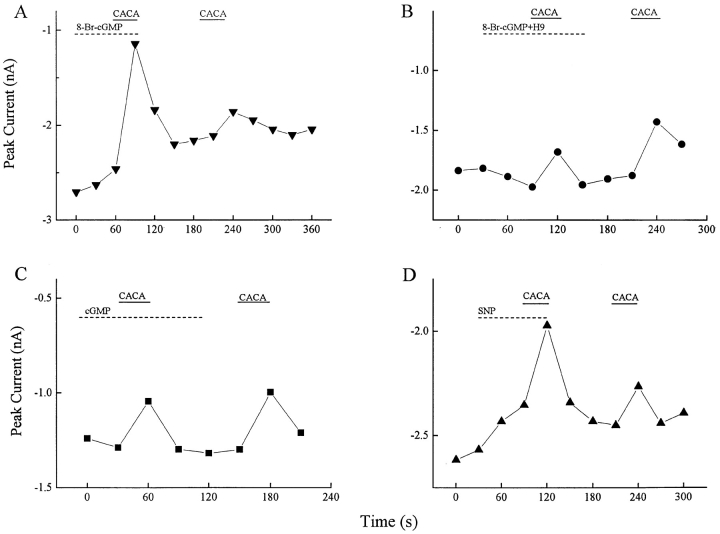
As noted, internal levels of GTP regulate the CACA-sensitive GABABR. Associated with this observation, GABAB-CACAR activation was significantly (P < 0.01) enhanced by sodium nitroprusside or by 8-bromo-cyclic GMP, as illustrated in Fig. 12. In these experiments, a voltage ramp was applied every 30 s and the peak inward barium current was measured. When 50 μM 8-bromo- cyclic GMP was superfused on the retinal slice (Fig. 12 A), it produced a modest reduction in the barium current. In the presence of 8-bromo-cGMP, 50 μM CACA produced a large reduction in the HVA barium current. After recovery, CACA alone was applied and caused a smaller reduction in the barium current. In ten cells, CACA in the presence of 8-bromo-cGMP reduced the barium current by 38 ± 4%, while CACA alone suppressed 12 ± 2% of this current. The same experiment was performed in the presence of 20 μM H-9, a blocker of guanylate kinase. Under these conditions the effect of CACA was not enhanced by 8-bromo-cGMP (Fig. 12 B). In ten cells, CACA alone suppressed 15 ± 3% of the barium current, but in the presence of H-9 and 8-bromo-cGMP only 11 ± 3% of the current was suppressed. As another control, the retina was superfused with membrane impermeant 100 μM cGMP. It did not enhance the effect of CACA in any of 16 cells tested. When the retina was superfused with 300 μM sodium nitroprusside, a generator of NO, it also produced a modest suppression of the peak calcium current and it enhanced the effect of CACA (Fig. 12 D). In seven cells, CACA alone produced a 6 ± 1% calcium current suppression, while in the presence of sodium nitroprusside CACA suppressed 21 ± 6% of the calcium current. NG-monomethyl-l-arginine, an inhibitor of nitric oxide synthase, was tested in four ganglion cells, but alone it did not suppress the effect of CACA (data not shown). These results indicate that nitric oxide stimulation of cGMP can up-regulate the CACA-sensitive GABA receptor, but that the NO system is not endogenously active under the conditions of our experiments.
Figure 12.
The GABAB-CACAR pathway is enhanced by nitric oxide. CACA's effect was enhanced when the retina was superfused with 8-bromo-cGMP or sodium nitroprusside. In each graph, the peak voltage-activated barium current was measured every 30 s and plotted. (A) While 50 μM 8-bromo-cGMP slightly reduced the barium current, 50 μM CACA in the presence of this second messenger analogue produced a much larger decrease in barium current than when applied alone (n = 22). (B) When a similar experiment was performed with the addition of 20 μM H-9, a cGMP kinase inhibitor, the effect of CACA was not enhanced (n = 10). (C) Addition of the impermeant 100 μM cGMP to the bath did not increase the CACA effect (n = 16). (D) When 300 μM sodium nitroprusside was added to the superfusate, the effect of CACA was enhanced (n = 6).
discussion
GABA Receptor Diversity in Ganglion Cells
This study reveals that amphibian ganglion cells contain two distinct metabotropic GABA receptors: GABAB-BACLOFENR and GABAB-CACAR. These neurons also possess two ionotropic GABA receptors, GABAAR and GABACR, which regulate chloride conductances (Zhang and Slaughter, 1995). Although we did not test for all of the GABARs on each ganglion cell, each receptor subtype was found on almost all neurons examined, indicating that most ganglion cells contain all four subtypes. The GABAAR is a rapid onset, fast desensitizing receptor, while the GABACR has slower activation kinetics but shows little desensitization (Qian and Dowling, 1993; Feigenspan et al., 1993; Amin and Weiss, 1994). Therefore, the GABAAR may be more influential in phasic inhibition while the GABACR may produce more tonic inhibition. A similar separation of function occurs between the two metabotropic GABARs. Both down-regulate calcium currents, but they affect different types of calcium channel, they act through different second messenger pathways, and they are differentially regulated. Furthermore, the GABAB-BACLOFENRs also activate a small potassium conductance (Slaughter and Bai, 1989; Zhang and Slaughter, personal communication), while the GABAB-CACARs do not. It fits the premise that the inhibitory GABAergic input to ganglion cells is eclectic, allowing a spectrum of inhibitory responses to the same transmitter. A preliminary report of a similar diversity of GABA receptors in turtle retinal ganglion cells (Liu and Lasater, 1994b ) suggests this is a general characteristic of these neurons. As discussed below, each receptor pathway can be differentially modulated, indicating that the relative weighting of this panoply of GABA inhibition is transformable.
Modulation of the GABAB-BACLOFENR Transduction Pathway
The action of GDP-β-S indicates that the GABAB-BACLOFENR acts through a G-protein pathway (Fig. 7), and prepulse experiments suggest that a G-protein subunit may directly modulate the calcium channel (Boland and Bean, 1993). Forskolin and internal cAMP reduced the effect of baclofen, but even relatively high doses of these agents did not eliminate baclofen's effect. Moreover, the effect of baclofen was reversed by prepulse facilitation while forskolin's action was unaltered by this protocol. Therefore, it is likely that PKA activates a second, parallel pathway that independently down-regulates the same channels affected by baclofen.
Blocking of calcium-calmodulin with calmidazolium, trifluoperazine, or W-7 largely suppressed GABAB-BACLOFENR action. None of these agents alone produced an obvious alteration in the calcium current. Subtle changes would be difficult to detect because all drugs were applied by internal dialysis and therefore were compared statistically to other neurons that served as a control. Calcium-calmodulin stimulates a number of pathways, including specific phosphatases and kinases. KN-62, a potent blocker of some calcium-calmodulin kinases, did not suppress the effect of baclofen. Blockers of calcium-calmodulin dependent phosphatases, such as cyclosporin A and microcystin, enhanced the effect of baclofen. Phosphatase inhibitors did not appear to have a direct effect on the calcium current, indicating that phosphorylation enhanced the GABAB-BACLOFENR pathway.
Thus, calmodulin-dependent phosphatases down-regulated the GABAB-BACLOFENR. But calmodulin antagonists reduced the effect of baclofen, indicating that calmodulin stimulation is required for GABAB-BACLOFENR activity. Therefore, another calmodulin pathway must be involved, possibly a calmodulin-dependent kinase. The ineffectiveness of KN-62 prevents clear identification of a calmodulin kinase, but the action of H-7 (coupled with the ineffectiveness of Rp-cAMP) could be interpreted as a block of a calmodulin-kinase pathway. While the precise calmodulin kinase pathway was not identified, these experiments suggest a regulatory scheme In which calmodulin modulates the activity of the GABA receptor by both up-regulation through kinase activation and down-regulation through phosphatase activation.
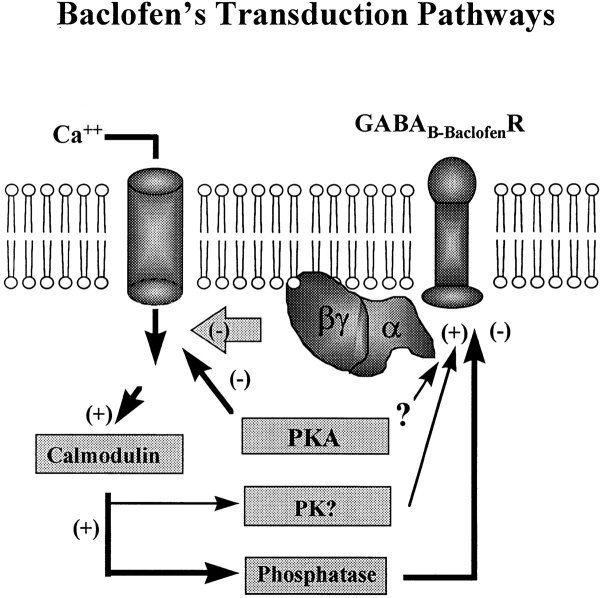
A tentative model of GABAB-BACLOFENR transduction consistent with our data (Fig. 13) proposes that baclofen down-regulates N-type calcium channels through a direct G-protein pathway. The GABAB-BACLOFENR is biphasically modulated by calmodulin: up-regulation of the receptor by calmodulin, probably through a kinase, and down-regulation through a calmodulin-dependent phosphatase. The balance between these two calmodulin-dependent enzymes would determine the phosphorylation state, and therefore the activity, of this transduction pathway. In addition, a parallel PKA pathway also down-regulates the same population of calcium channels.
Figure 13.
A tentative model of the baclofen transduction pathway. Baclofen reduces the voltage-activated calcium current via a direct G-protein interaction with N-type calcium channels. Through a parallel mechanism, the cAMP pathway also reduces current through this channel. A calcium-calmodulin (Ca/CaM)-dependent kinase (PK) is required for GABAB-BACLOFENR activity. Calmodulin-dependent phosphatases (PP) down-regulate this receptor. Calcium entry through the N-type channel may activate an autoregulatory negative feedback by stimulating the calmodulin kinase.
One ramification of the model is that the calcium-calmodulin system provides a feedback loop between the calcium channel and the GABAB-BACLOFENR. The GABAB-BACLOFENR pathway does not appear to require calcium from internal stores (based on the heparin and ruthenium red data). Therefore, calcium influx through the voltage-sensitive calcium channel may stimulate calmodulin, which up-regulates the GABAB-BACLOFENR. A check on this negative feedback would be the phosphatase system, which turns off the baclofen receptor. This mechanism could help to regulate the internal calcium concentration. The model implies that the calmodulin activation of kinases and phosphatases have different calcium sensitivities or that there is higher gain in the kinase circuit.
Modulation of GABAB-CACAR Action
Considering the variety of GABA receptors in ganglion cells, changes in the weighting between different receptors can serve to alter the postsynaptic response to the same presynaptic GABAergic inputs. Second messengers can modulate each GABA receptor. We found that internal dialysis with ATP or GTP shifted the relative effectiveness of the GABAB-BACLOFENR and GABAB-CACAR, respectively. Related to this effect, 8-bromo-cGMP or sodium nitroprusside augmented the effects of CACA application. This implies that NO stimulation of guanylate cyclase can enhance the effectiveness of the GABAB-CACAR-activated second messenger cascade. However, NO antagonists alone did not decrease the effect of CACA, indicating that NO production was normally low under the conditions of our experiments. Liepe et al. (1994) found that many cells in the tiger salamander retina have the ability to synthesis NO. Amacrine, ganglion, and Müller cells all have this capability and are positioned to affect the ganglion cell GABAB-CACAR.
Many regulatory mechanisms also produce differential effects on ionotropic GABARs. For example, protein kinase C reduces GABACR and enhances GABAAR currents (Dong and Werblin, 1994; Feigenspan and Bormann, 1994; Wellis and Werblin, 1995). Similarly, low micromolar concentrations of zinc can reduce the GABACR current but enhance the GABAAR current (Chappell et al., 1995). Zinc is believed to be synaptically released by photoreceptors (Wu et al., 1993). Overall, these modulatory mechanisms can be employed to adjust the balance of inhibitory action produced by the four GABA receptor subtypes and suggests that the effects of the ionotropic receptors are complementary, as are the metabotropic receptors.
In summary, the range of GABA receptors found on ganglion cells coupled with the ability to modulate their effects extends the processing capabilities at this final step in the retinal network. Modulation of inhibition can be a mechanism to shift the balance of weighted inputs or postsynaptic processing in the retina's output neurons.
Acknowledgments
We thank Ciba-Geigy for the generous gifts of baclofen and CGP35348.
Footnotes
This research was supported by National Eye Institute grant R01-05725.
Dr. Jian Zhang's present address is Department of Ophthalmology and Visual Science, University of Texas Medical Center, Houston, TX 77030.
Abbreviations used in this paper: CACA, cis-aminocrotonic acid; GABA, γ-aminobutyric acid; GDP-β-S, guanosine 5′-O-(2-thiodiphosphate); HVA, high voltage–activated.
references
- Amin J, Weiss DS. Homomeric p1 GABA channels: activation properties and domains. Receptors Channels. 1994;2:227–236. [PubMed] [Google Scholar]
- Bindokas VP, Ishida AT. (-)-Baclofen and gamma-aminobutyric acid inhibit calcium currents in isolated retinal ganglion cells. Proc Natl Acad Sci USA. 1991;88:10759–10763. doi: 10.1073/pnas.88.23.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland LM, Bean BP. Modulation of N-type calcium channels in bullfrog sympathetic neurons by luteinizing hormone-releasing hormone: kinetics and voltage dependence. J Neurosci. 1993;13:516–533. doi: 10.1523/JNEUROSCI.13-02-00516.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell V, Berrow NS, Fitzgerald EM, Brickley K, Dolphin AC. Inhibition of the interaction of G protein G(o) with calcium channels by the calcium channel beta-subunit in rat neurones. J Physiol (Lond) 1995;485:365–372. doi: 10.1113/jphysiol.1995.sp020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell RL, Qian H, Malchow RP, Ripps H. Novel action of zinc on GABA receptors of skate bipolar cells. Invest Ophthalmol Visual Sci (Suppl) 1995;36:S215. [Google Scholar]
- Cohen BN, Fain GL, Fain MJ. GABA and glycine channels in isolated ganglion cells from goldfish retina. J Physiol (Lond) 1989;418:53–82. doi: 10.1113/jphysiol.1989.sp017828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting GR, Lu L, O'Hara B, Kasch LM, Montrose-Rafizadeh C, Donovan DM, Schimada S, Antonarakis SE, Guggino WB, Uhl GR, Kazazian HH., Jr Cloning of the γ-amino- butyric acid (GABA) ρ1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc Natl Acad Sci USA. 1991;88:2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C-J, Picaud SA, Werblin FS. GABA transporters and GABAC-like receptors on catfish cone- but not rod-driven horizontal cells. J Neurosci. 1994;14:2648–2658. doi: 10.1523/JNEUROSCI.14-05-02648.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C-J, Werblin FS. Dopamine modulation of GABACreceptor function in an isolated retinal neuron. J Neurophysiol. 1994;71:1258–1260. doi: 10.1152/jn.1994.71.3.1258. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. Modulation of GABACreceptors in rat retinal bipolar cells by protein kinase C. J Physiol. 1994;481:325–330. doi: 10.1113/jphysiol.1994.sp020442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Wässle H, Bormann J. Pharmacology of GABA receptor Cl−channels in rat retinal bipolar cells. Nature (Lond) 1993;361:159–162. doi: 10.1038/361159a0. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grunert U, Fritschy JM, Stephenson A, Mohler H, Wässle H. GABAA receptor subunits have differential distributions in the rat retina: in situhybridization and immunohistochemistry. J Comp Neurol. 1995;353:553–571. doi: 10.1002/cne.903530407. [DOI] [PubMed] [Google Scholar]
- Hille, B. 1992. Ionic Channels in Excitable Membranes. 2nd ed. Sinauer Associates Inc. Sunderland, MA. 607.
- Ishida AT, Cohen BN. GABA-activated whole-cell currents in isolated retinal ganglion cells. J Neurophysiol. 1988;60:381–396. doi: 10.1152/jn.1988.60.2.381. [DOI] [PubMed] [Google Scholar]
- Kerr DI, Ong J, Johnston GAR, Abbenante J, Prager RH. 2-Hydroxy-saclofen: an improved antagonist at central and peripheral GABABreceptors. Neurosci Lett. 1988;92:92–96. doi: 10.1016/0304-3940(88)90748-3. [DOI] [PubMed] [Google Scholar]
- Liepe BA, Stone C, Koistinaho J, Copenhagen DR. Nitric oxide synthase in Müller cells and neurons of salamander and fish retina. J Neurosci. 1994;14:7641–7654. doi: 10.1523/JNEUROSCI.14-12-07641.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lasater EM. Calcium currents in turtle retinal ganglion cells. II. Dopamine modulation via a cyclic AMP-dependent mechanism. J Neurophysiol. 1994a;71:743–752. doi: 10.1152/jn.1994.71.2.743. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lasater EM. Multiple GABA receptors of ganglion cells in the turtle retina. Invest Ophthalmol Visual Sci (Suppl) 1994b;35:2154. [Google Scholar]
- Lukasiewicz PD, Werblin FS. A slow inactivating potassium current truncates spike activity in ganglion cells of the tiger salamander retina. J Neurosci. 1988;8:4470–4481. doi: 10.1523/JNEUROSCI.08-12-04470.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz PD, Werblin FS. A novel GABA receptor modulates synaptic transmission from bipolar to ganglion and amacrine cells in the tiger salamander retina. J Neurosci. 1994;14:1213–1223. doi: 10.1523/JNEUROSCI.14-03-01213.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire G, Maple B, Lukasiewicz P, Werblin FS. Gamma-aminobutyrate type B receptor modulation of L-type calcium channel current at bipolar cell terminals in the retina of the tiger salamander. Proc Natl Acad Sci USA. 1989;86:10144–10147. doi: 10.1073/pnas.86.24.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G, Ayoub GS, Heidelberger R. Presynaptic inhibition by GABA is mediated via two distinct GABA receptors with novel pharmacology. J Neurosci. 1994;14:1079–1090. doi: 10.1523/JNEUROSCI.14-03-01079.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RF, Frumkes TE, Slaughter M, Dacheux RF. Physiological and pharmacological basis of GABA and glycine action on neurons of mudpuppy retina. II. Amacrine and ganglion cells. J Neurophysiol. 1981;45:783–804. doi: 10.1152/jn.1981.45.4.783. [DOI] [PubMed] [Google Scholar]
- Müller F, Boos R, Wässle H. Actions of GABAergic ligands on brisk ganglion cells in the cat retina. Visual Neurosci. 1992;9:415–425. doi: 10.1017/s0952523800010828. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Pan Z-H, Lipton SA. Multiple GABA receptor subtypes mediate inhibition of calcium influx at rat retinal bipolar cell terminals. J Neurosci. 1995;15:2668–2679. doi: 10.1523/JNEUROSCI.15-04-02668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z-H, Slaughter MM. Comparison of the actions of glycine and related amino acids on isolated third order neurons from the tiger salamander retina. Neuroscience. 1995;64:153–164. doi: 10.1016/0306-4522(94)00399-p. [DOI] [PubMed] [Google Scholar]
- Qian H, Dowling JE. Novel GABA responses from rod-driven retinal horizontal cells. Nature (Lond) 1993;361:162–164. doi: 10.1038/361162a0. [DOI] [PubMed] [Google Scholar]
- Qian H, Dowling JE. Pharmacology of novel GABA receptors found on rod horizontal cells of the white perch retina. J Neurosci. 1994;14:4299–4307. doi: 10.1523/JNEUROSCI.14-07-04299.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter MM, Bai S-H. Differential effects of baclofen on sustained and transient cells in the mudpuppy retina. J Neurophysiol. 1989;61:374–381. doi: 10.1152/jn.1989.61.2.374. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Miyoshi S, Kaneko A. Two components of GABA-induced currents in catfish retinal horizontal cells. Jpn J Physiol. 1994;44:S141–144. [PubMed] [Google Scholar]
- Tian N, Slaughter MM. Pharmacology of the GABABreceptor in amphibian retina. Brain Res. 1994;660:267–274. doi: 10.1016/0006-8993(94)91299-8. [DOI] [PubMed] [Google Scholar]
- Wellis DP, Werblin FS. Dopamine modulates GABACreceptors mediating inhibition of calcium entry into and transmitter release from bipolar cell terminals in tiger salamander retina. J Neurosci. 1995;15:4748–4761. doi: 10.1523/JNEUROSCI.15-07-04748.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS. Transmission along and between rods in the tiger salamander retina. J Physiol (Lond) 1978;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S-M. Synaptic connections between neurons in living slices of the larval salamander retina. J Neurosci Methods. 1987;20:139–149. doi: 10.1016/0165-0270(87)90046-x. [DOI] [PubMed] [Google Scholar]
- Wu SM, Qiao X, Noebels JF, Yang XL. Localization and modulatory actions of zinc in vertebrate retina. Vision Res. 1993;33:2611–2616. doi: 10.1016/0042-6989(93)90219-m. [DOI] [PubMed] [Google Scholar]
- Zhang J, Slaughter MM. Preferential suppression of the ON pathway by GABACreceptors in the amphibian retina. J Neurophysiol. 1995;74:1583–1592. doi: 10.1152/jn.1995.74.4.1583. [DOI] [PubMed] [Google Scholar]