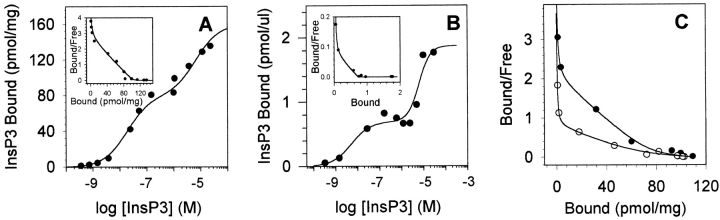
Figure 3.
InsP3 binding reveals three binding sites. (A) Saturation-binding isotherm of InsP3 binding to cerebellar reticular vesicles. InsP3 binding was conducted in the absence of Ca with InsP3 concentrations varying between 0.4 nM and 30 μM. Inset shows the Scatchard analysis. Data were fit assuming the presence of three binding sites for InsP3. Four different vesicle preparations were used; one experiment is displayed. In the absence of Ca, the K d's for the vesicle preparations are 0.47 ± 0.19 nM, 54 ± 5 nM, and 10.2 ± 3.2 μM; x̄ ± SEM, n = 4. (B) Saturation-binding isotherm of InsP3 binding to the type 1 InsP3 receptor purified by heparin-agarose chromatography. Inset shows the Scatchard analysis. Similar results were obtained with a receptor purified from cerebellar microsomes by immunoprecipitation using a type 1-specific antibody (C-19). The purity of the receptor, as assessed from InsP3 binding, was 94% (InsP3 binding was 3,612 pmol/ mg for the 50-nM site, compared with a theoretical maximum binding of 3,846 pmol/mg for a molecular weight of 260 kD). In this panel, the abscissa is expressed as picomoles per microliter of purified InsP3 receptor. K d's for this preparation are 0.5 nM, 29 nM, and 6.8 μM. For comparison, one preparation of InsP3 receptor purified by immunoprecipitation generated K d's of 0.1 nM, 35 nM, and 12 μM. (C) Scatchard analysis of InsP3 binding in the absence of Ca (•) and 10 μM free Ca (○). Increasing the free Ca results in a decrease in the apparent affinity of InsP3 binding from 35 nM in the absence of Ca to 91 nM in the presence of 10 μM Ca without altering binding to the 1-nM or 10-μM sites. The Ca-induced decrease in InsP3 affinity of the 50-nM site was completely reversed upon addition of EGTA.