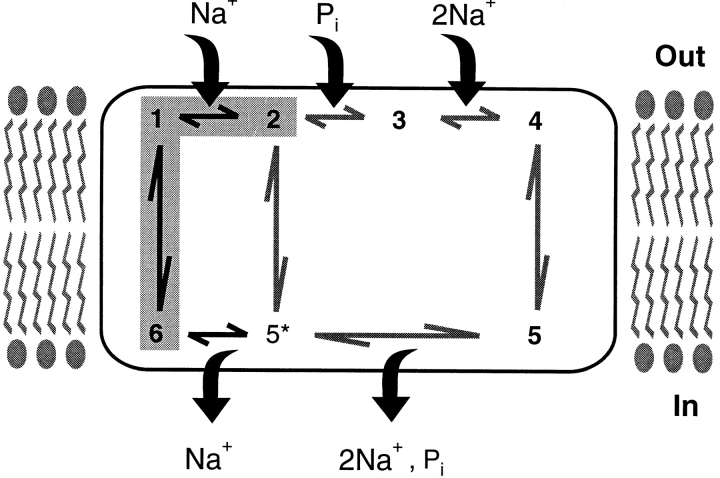
Figure 9.
A state diagram representation of an ordered binding model for NaPi-2. Voltage-dependent partial reactions identified by pre–steady state experiments (translocation of the unloaded carrier, 6 ⇔ 1; binding of the first Na+, 1 ⇔ 2) are shown shaded. The empty cotransporter is assumed to have a valency of −1, but this charge translocates through a fraction of the transmembrane field to account for an apparent valency <1 derived from fits using Eq. 2. Both the slippage (2 ⇔ 5*) pathway and translocation of the fully loaded carrier (4 ⇔ 5) are assumed to be electroneutral. Depending on the availability of substrate and membrane potential, accessibility to the substrate binding sites favors either the cis (states 1, 2, 3, 4) or trans (states 5, 5*, 6) side of the membrane. Under normal physiological conditions with V < 0, the apparent transport cycle proceeds clockwise around the loop. For V < 0, and low internal Na+, external Na+ binding is facilitated on the cis side due to the outward translocation of −ve charge associated with transition 6 ⇔ 1. This allows one Na+ ion to move to its binding site within the transmembrane field, leading to an increasing of the affinity of the transporter for Pi (assumed to be predominantly divalent at neutral pH), which then binds (2 ⇔ 3) in a voltage-independent manner. Then follows a further voltage-independent step involving the binding of two Na+ ions (3 ⇔ 4) to give electroneutrality. The fully loaded carrier is now able to translocate (4 ⇔ 5), release substrates on the trans side, and return to state 6. A net inward charge movement of +1 electronic units occurs per cycle, which is manifested as Ip. Except for slippage, the order of binding/release of substrates on the cytosolic side cannot be determined using the intact oocyte preparation and, therefore, the cytosolic release of cotransported Pi, together with two Na+ ions, is lumped as one reaction (5 ⇔ 5*).