Figure 1.
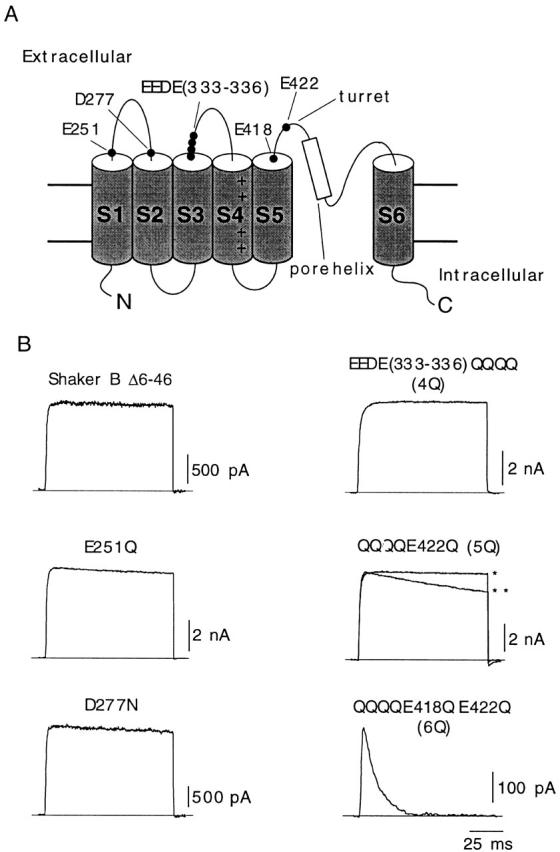
Differential effects of neutralization of charges in extracellular dicarboxylic residues of Shaker K+ channels. (A) Scheme of the transmembrane topology of the α subunit of the K+ channel with indication of the residues mutated in this study. (B) Representative recordings of outward K+ currents from six different types of channels studied. In all cases, the membrane was depolarized from −80 to +20 mV during 100-ms depolarizations, and external K+ was 2.7 mM. Two different recordings corresponding to channel 5Q are superimposed to illustrate the variability of C-type inactivation in this mutant (*time constant, 1,970 ms; **time constant, 542 ms). Current amplitude is scaled to match the value of the current in the trace indicated with the single asterisk.
