Figure 1.
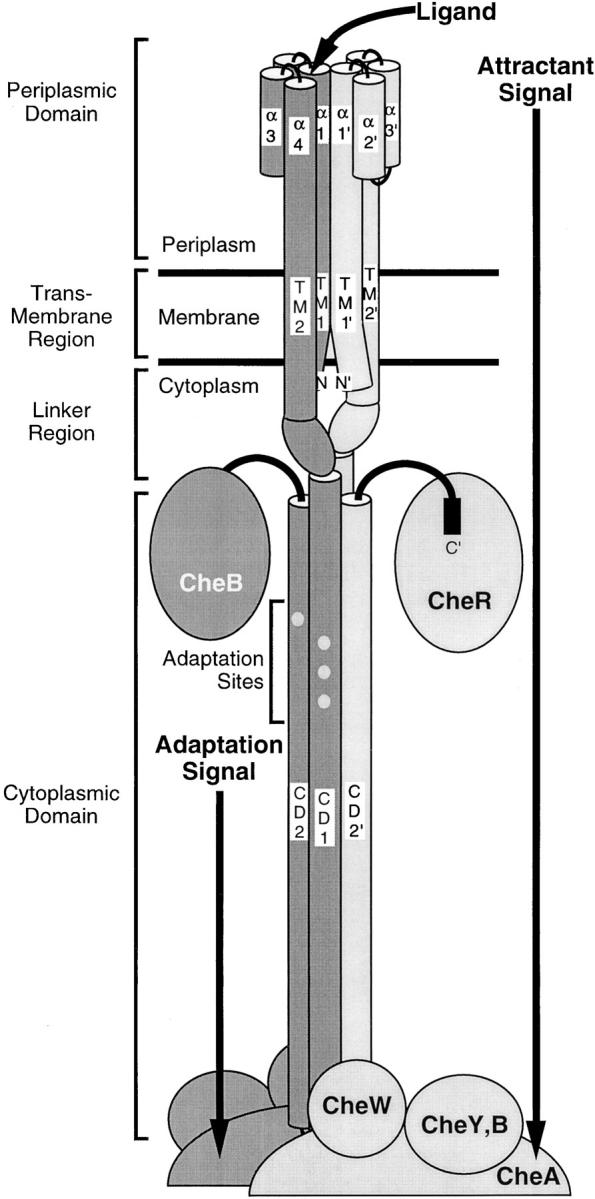
Schematic model of the full-length homodimeric membrane bound receptor in the associated signaling complex (Falke and Hazelbauer 2001). Cylinders represent helical domains determined by crystallographic or cysteine and disulfide scanning methods. The two 60-kD receptor subunits are differentially shaded. The receptor provides a scaffold for the formation of a large super-molecular signaling complex. Proposed docking sites for the methyltransferase CheR, methylesterase CheB, histidine kinase CheA, coupling protein CheW and motor response regulator CheY are shown. The core ternary signaling complex consisting of the dimeric receptor, dimeric CheA, and CheW molecules is stable both in the presence and absence of attractant. Filled circles represent the four adaptation sites on each receptor subunit. Binding of attractant at the periplasmic domain of the receptor and modification of the cytoplasmic adaptation sites serve to modulate the kinase activity of the signaling complex.
