Figure 6.
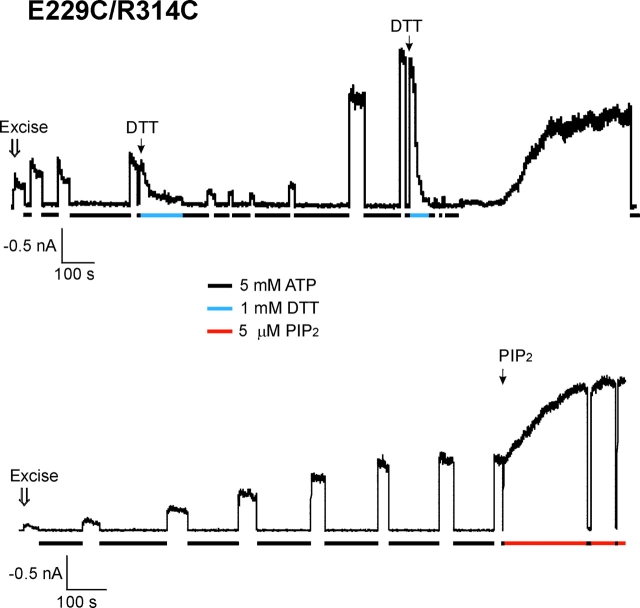
Disulfide bond formation between C229 and C314 in the E229C/R314C mutant channel stabilizes channel opening. Currents recorded from two separate membrane patches containing the E229C/R314C mutant channels. Upon patch excision into K-INT/EDTA, the channel exhibited low open probability and mild inactivation. However, the current gradually increased in amplitude and stability. Exposure of the patch to 5 mM ATP (indicated by the black bars underneath the current traces) accelerated the current reactivation process. (Top trace) Application of 1 mM DTT (in K-INT/EDTA, indicated by the gray bar) after current stabilization induced rapid inactivation of the channel in a reversible manner, indicating that the current stabilization was due to spontaneous disulfide bond formation. (Bottom trace) After the current has reached the maximum, application of 5 μM PIP2 (see materials and methods) further stimulated channel activity by ∼2-fold.