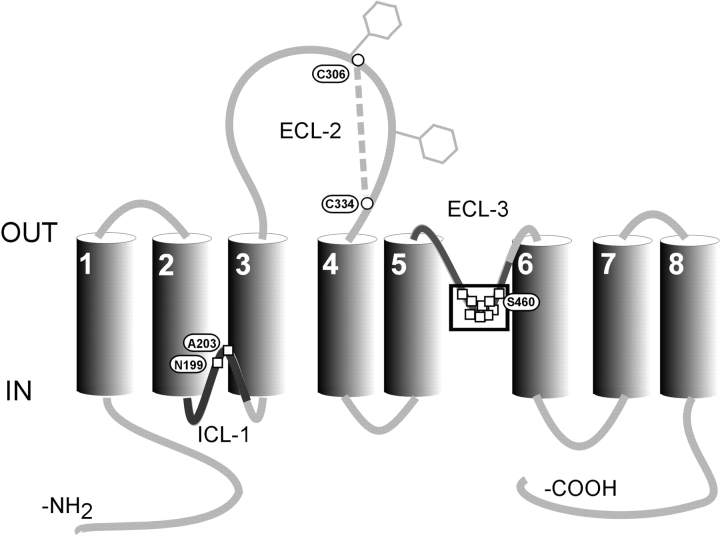
Figure 1.
Secondary topology of NaPi-IIa showing the eight putative transmembrane domains based on hydrophobicity analysis and topology studies. Two N-glycosylation sites in the second extracellular loop are indicated by hexagons and a cysteine bridge has been identified between Cys-306 and Cys-334. Squares indicate sites where cysteine substitution and/or exposure to MTS reagents alter transport function. The boxed residues in ECL-3 represent a region that forms a proposed 2.5 turn α-helix (Lambert et al., 2001). The bold regions of ICL-1 and ECL-3 represent two stretches of ∼50 amino acids that show high similarity (Kohler et al., 2002).