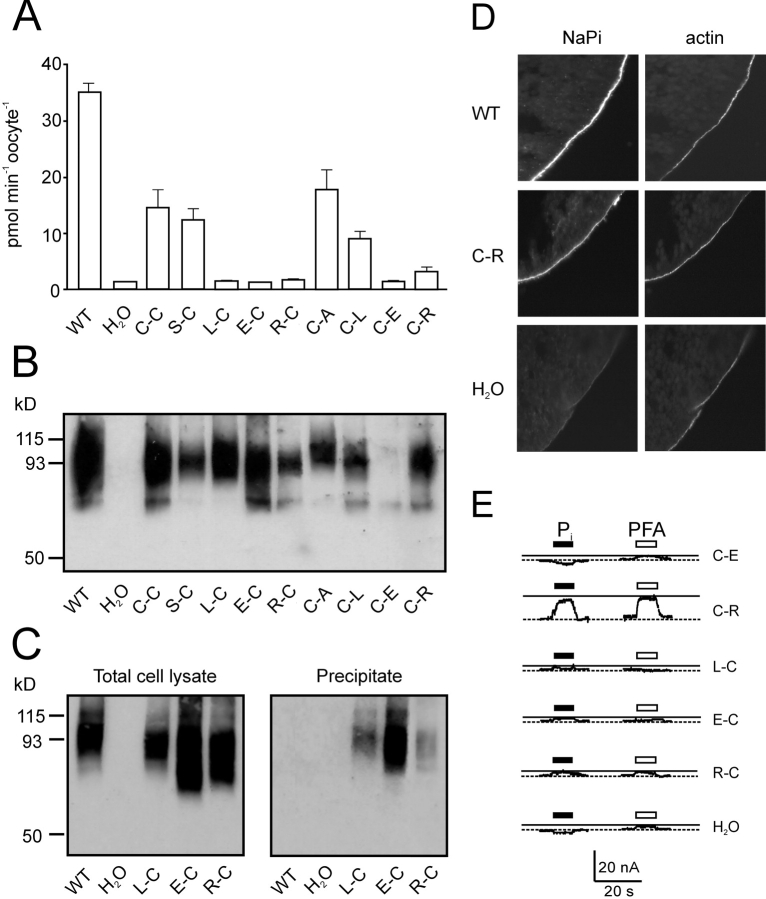
Figure 4.
Characterization of other double mutants. (A) 32Pi uptake of cells injected with WT and mutant cRNA and water-injected oocytes. The bars represent the mean ± SEM (n = 8). Only the WT, C-C, S-C, C-A, and C-L mutants gave statistically significant 32Pi uptake compared with control oocytes (Students t test, P < 0.05). (B) Protein expression of mutants in oocytes. Western blot of whole cell lysate from a pool of 5 oocytes injected with cRNA coding for the indicated mutants as well as the wild-type (WT) and water-injected oocytes as controls. NaPi-IIa was visualized using an antibody raised against the rat NaPi-IIa -NH2 terminus. (C) Surface biotinylation of mutant constructs. Western blot obtained from a pool of eight oocytes injected either with water, WT or the indicated mutants cRNA. 5 μl of whole oocyte lysate (left) or 15 μl of streptavidin-precipitate of MTSEA-biotinylated oocytes expressing the indicated mutants or WT and water injected oocytes (right). Band at 93 kD confirms that the labeled protein is NaPi-IIa. (D) Immunocytochemical detection of WT NaPi-IIa and C-C mutant and β-actin in cryosections of Xenopus oocytes. Oocytes injected with H2O, or cRNA for WT, or C-C mutant were cut and cryosections labeled with a rabbit polyclonal antibodies directed against the NH2 terminus of NaPi-IIa and with phalloidin-Texas red. Specific immunostaining appears in the oocyte surface as indicated by the actin signal. No specific NaPi-IIa staining was seen for H2O injected oocytes. Magnification, 60×. (E) Representative electrogenic response to 1 mM Pi and 3 mM PFA for double mutants. Traces show Pi and PFA-induced currents obtained from oocytes expressing the mutated constructs, measured at 1 mM Pi (filled bar) or 3 mM PFA (open bar) in ND100 and V h = −50 mV. Test substrates were applied for 20 s during period indicated by bars.