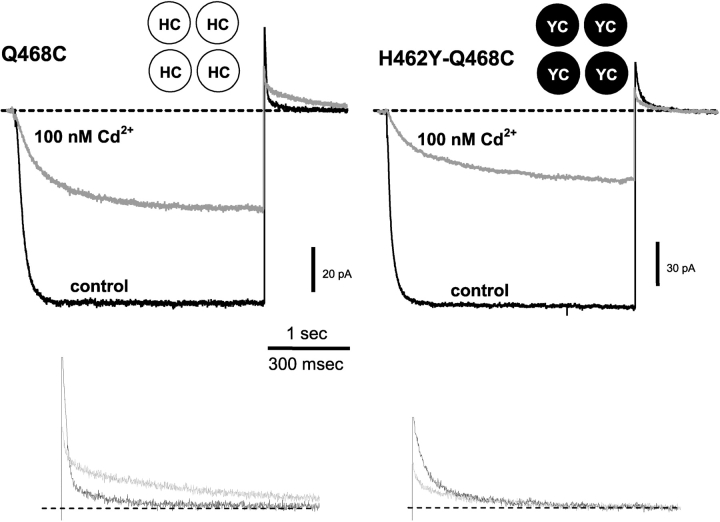
Figure 5.
Cd2+ slows closing of 468C channels through an interaction with H462. Current from inside-out patches containing 468C channels (left) and the H462Y-Q468C double mutant (right). Channels were held closed at +50 mV, stepped to −100 mV for 3 s, then stepped back to +50 mV. 100 nM Cd2+ slows both opening and closing when H462 is present (left). The H462Y mutation eliminates the slowed closing (right). The schematic insets indicate the amino acids at positions 462 and 468 in each of the four subunits. Lower traces show the tail currents on a larger scale (control = black traces, Cd2+ = gray traces).