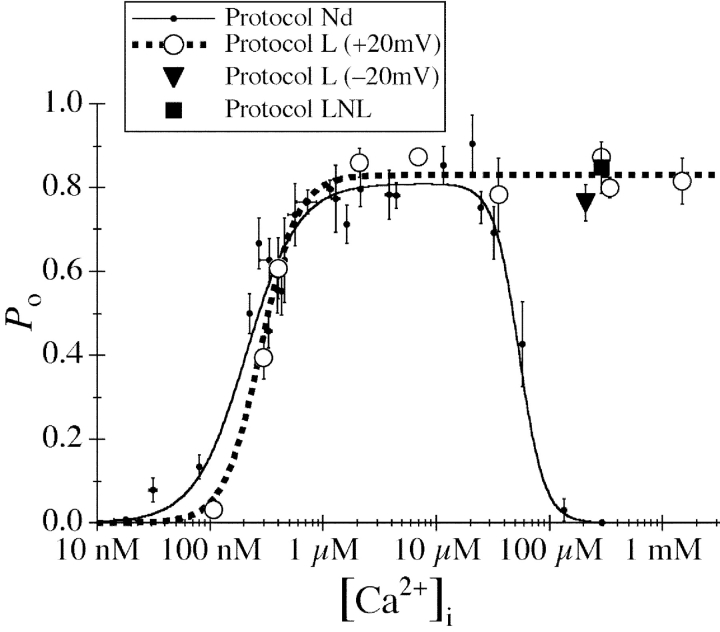
Figure 5.
[Ca2+]i dependencies of the channel P o of the InsP3R in oocyte nuclei isolated using various protocols (Nd, L, and LNL) and applied potentials (±20 mV) as tabulated. All pipette solutions used contained 10 μM InsP3. The dashed curve is a simple activating Hill equation fit for the data from nuclei isolated with protocol L (large open circles). For comparison, the biphasic Hill equation fit (continuous curve) for the data points from nuclei isolated directly into NCaS bath (small filled circles) obtained in a previous study (Mak et al., 1998) are also shown. The InsP3R channel P o was lower in ULCaS than in NCaS at [Ca2+]i ≈ 100 nM. It is possible that this reflects some intrinsic properties of the InsP3R after exposure to the low bath [Ca2+]. Alternately, this may only be an artifact as a result of the movement of free Ca2+ ion across the open channel. With pipette [Ca2+]i ≈ 100 nM, when the oocyte nucleus was in NCaS ([Ca2+] = 400–500 nM), the Nernst reversal potential for Ca2+ ions was ∼35 mV so Ca2+ ions moved across the open InsP3R channel from the lumenal side to the cytoplasmic side despite an applied transmembrane voltage of 20 mV. This could cause the effective [Ca2+]i at the activating Ca2+-binding sites on the cytoplasmic side of the channel to be higher than the free [Ca2+] in the bulk of the pipette solution if the Ca2+-binding sites are close enough to the ion conducting pore. Conversely, when the nucleus was in ULCaS ([Ca2+] < 5 nM), Ca2+ ions moved across the open InsP3R channel in the opposite direction, down the electrical and chemical gradients, possibly lowering the effective [Ca2+]i at the Ca2+-binding sites. In [Ca2+]i < 250 nM, the mean open channel duration (<τo>) of the InsP3R increases with [Ca2+]i (Mak and Foskett, 1998). Therefore, if Ca2+ flux across the open InsP3R channel caused the effective [Ca2+]i at the activating Ca2+-binding sites to deviate from the free [Ca2+] in the bulk pipette solution, then channels in NCaS bath would have longer <τo> and higher channel P o than those in ULCaS bath, as observed. On the other hand, in [Ca2+]i > 300 nM, <τo> does not exhibit any dependence on [Ca2+]i although the mean closed channel duration (<τc>) is still affected by [Ca2+]i (Mak and Foskett, 1998). Deviation of effective [Ca2+] at the Ca2+-binding sites from the bulk free [Ca2+] would dissipate quickly by diffusion once the channel closed and therefore would not affect <τc>. Thus, there would be no difference between the observed P o of InsP3R in ULCaS and NCaS bath in [Ca2+]i > 300 nM, as observed.