Figure 4.
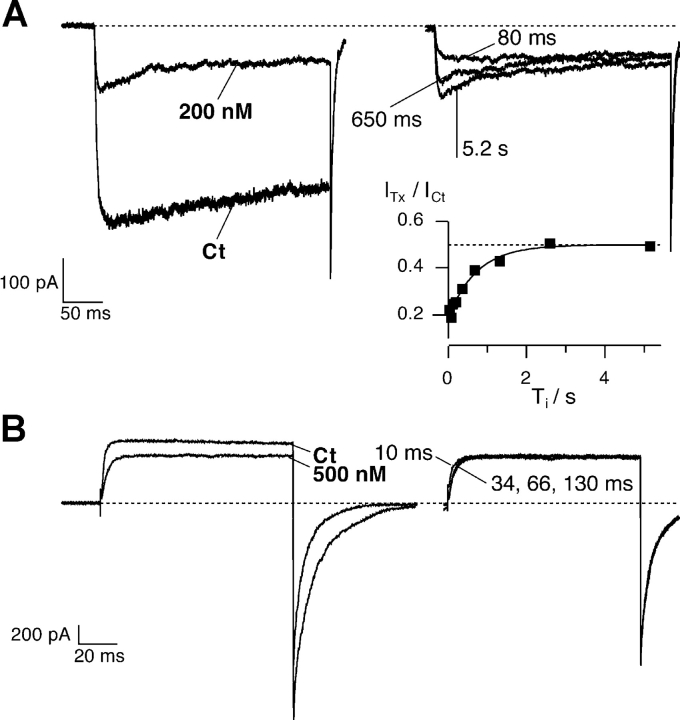
Relaxation of κ-PVIIA binding to closed Shaker-Δ channels after a depolarizing pulse. Outside-out patch clamp experiments performed in symmetrical 115 mM KCl (A) or 115 mM RbCl (B; same experiment as in Fig. 2). Superimposed records of responses to double pulse stimulation before and after the addition to the bath respectively of 200 nM and 500 nM κ-PVIIA. Each stimulation consisted of a conditioning pulse to VP, followed with a variable pulse interval, Ti, by an identical test pulse: (A) duration conditioning pulse = 300 ms, VP = −20 mV, VH = −100 mV, Ti shown 80 and 680 ms, and 5.2 s; (B) duration conditioning pulse = 100 ms, VP = +40 mV, VH = −100 mV, Ti shown 10, 34, 66, and 130 ms. (A) Symmetrical 115 mM KCl. The conditioning pulse induces an increase of toxin block that appears as a hump in the first response; in the second response for small Ti values no peak is observed. (A, inset) Amplitude of the second response at early time normalized to the control response and plotted as a function of Ti. The continuous line is the best exponential fit with τ = 720 ms, and the dotted horizontal line corresponds to the asymptotic value of U = ITx/ICt = 0.50, giving KC = 200 nM. From U and τ from Eqs. 2 and 3, we estimate kC off = 0.69 s−1 and kC on = 3.5 μM−1s−1. (B) Symmetrical 115 mM RbCl. The toxin equilibrates very fast with the closed state and 60 ms is enough to reach the equilibrium: the responses to the second pulse are indistinguishable after 66 ms. The process of toxin equilibration with the fraction of channels that are in the closed state has the same time scale of the deactivation process. Consequently, a kinetic analysis of the reblock is not possible.