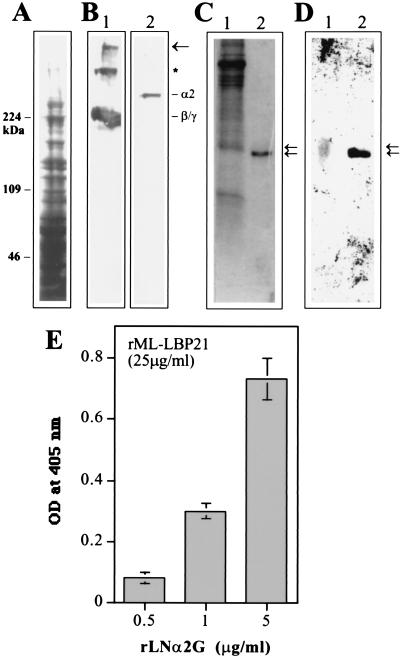
Figure 4.
rML-LBP21 binds to the endogenous peripheral nerve laminin. (A) Coommassie blue-stained SDS/polyacrylamide gel of the pH 12.0 extracts of the bovine PNMF. Molecular mass markers (Mwt) in kDa are indicated on the left. (B) Corresponding immunoblot reacted with polyclonal anti-laminin antibody (lane 1) and monoclonal anti-laminin α2 chain antibody (lane 2). The high-molecular-mass band indicated by the arrow corresponds to the laminin heterotrimer that was not dissociated by SDS/PAGE. The band indicated by ∗ appears to correspond to the heterodimer composed of β1 and γ1 chain that were not dissociated by SDS. Also shown are the laminin β1, γ1 (lane 1), and α2 chains (lane 2). (C and D) The nitrocellulose transfers of rML-LBP21 and the M. leprae cell wall fraction were incubated with the pH 12.0 extracts of the peripheral nerve membrane fraction. (D) Bound peripheral nerve laminin was detected by polyclonal anti-laminin antibody. Arrows indicate the peripheral nerve laminin bound to native cell wall ML-LBP21 (lane 1) and r ML-LBP21 (lane 2) with slight difference in molecular masses. (C) Corresponding Coommassie blue-stained SDS/polyacrylamide gel containing M. leprae cell wall fraction and rML-LBP21. Arrows indicate to show the corresponding laminin-reactive bands. (E) Binding of rML-LBP21 to the rG domain of laminin α2 chain in ELISA. Plates coated with 25 μg/ml of rML-LBP21 were incubated with increasing concentration of rLNα2G domain and detected by affinity-purified antibody against rLNα2G.