Abstract
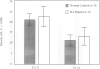
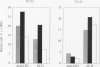
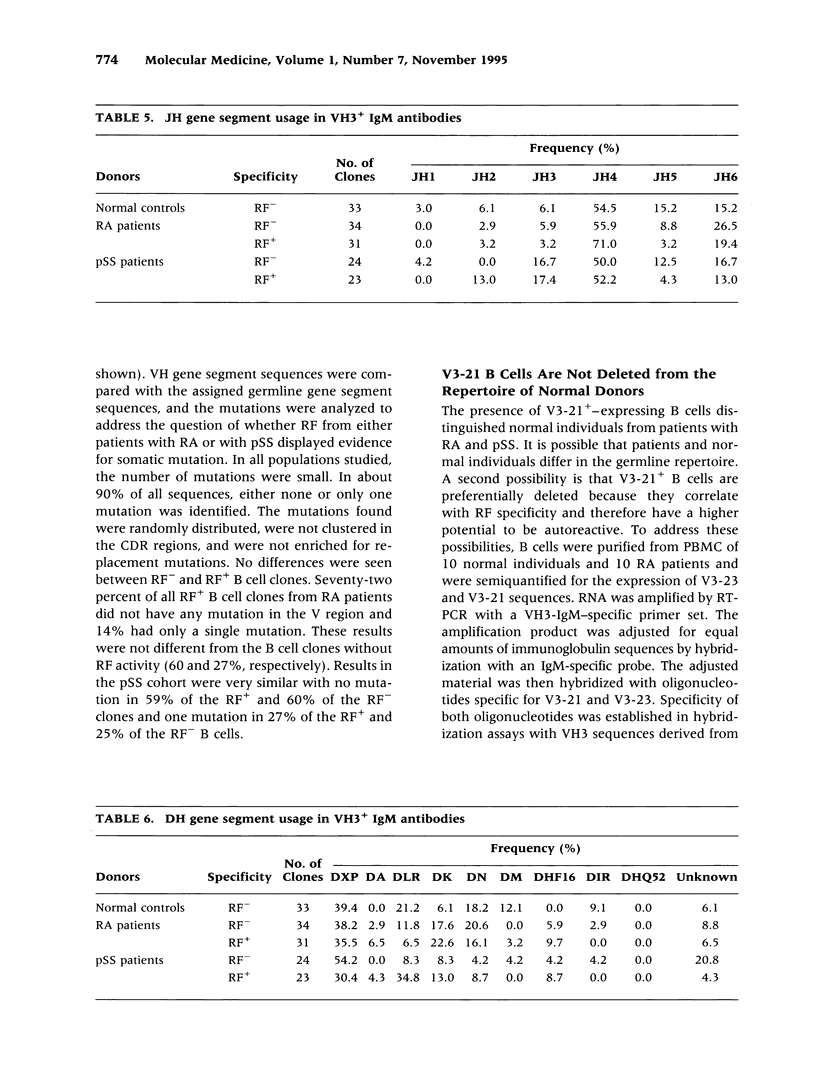
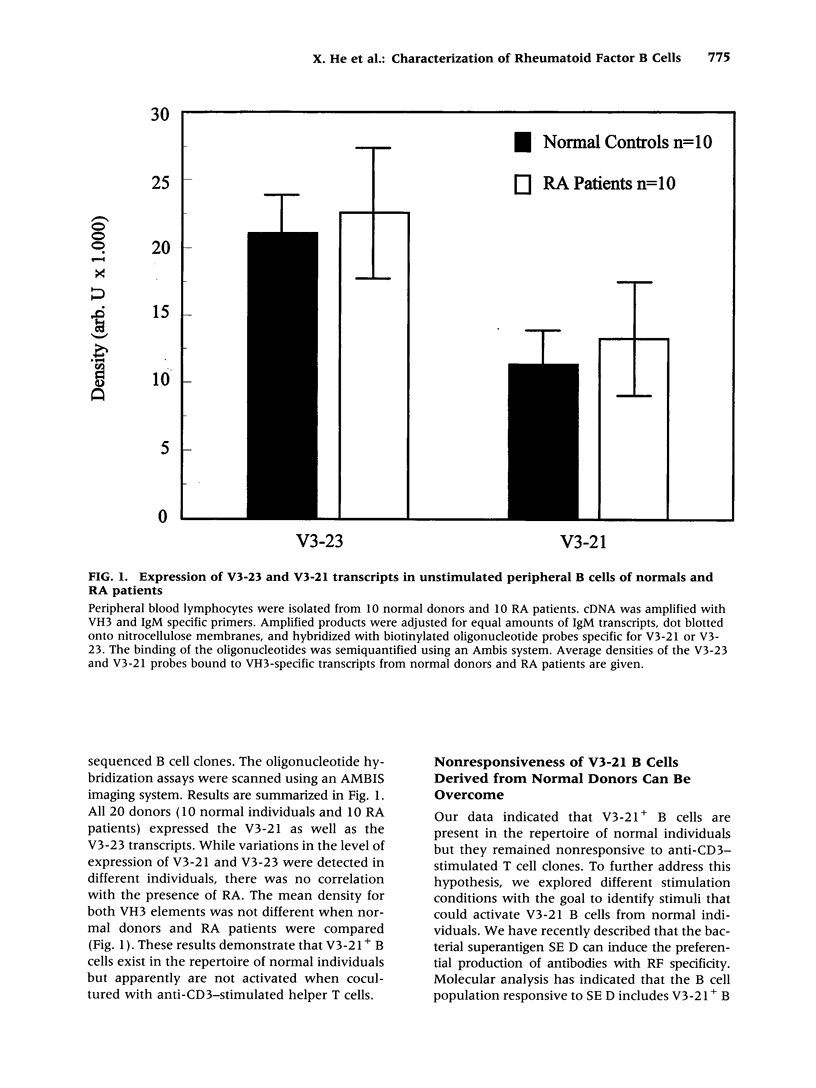
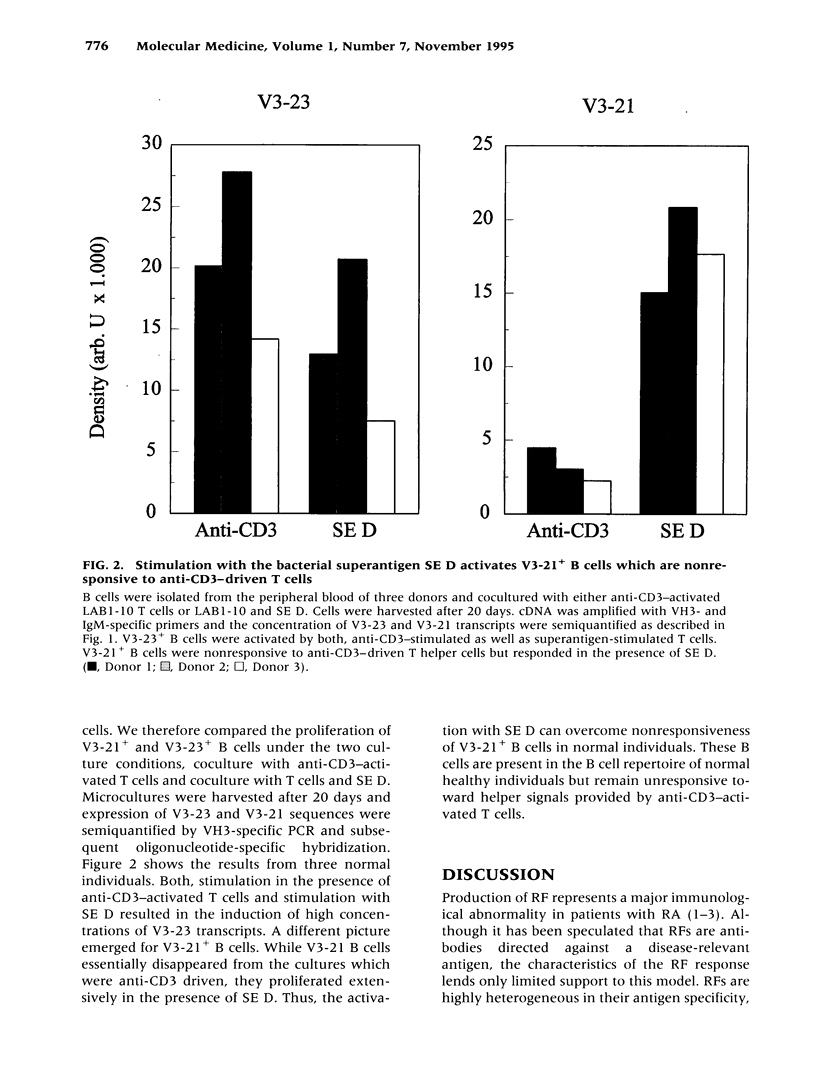
BACKGROUND: Rheumatoid factor (RF) is a characteristic but not pathognomic feature in patients with rheumatoid arthritis (RA). It is unknown whether the repertoire of immunoglobulin genes utilized by RF+ B cells of RA patients is unique and whether RF+ B cells in normal individuals are silenced or deleted. MATERIALS AND METHODS: Clonal B cell populations were established from the peripheral blood of normal donors (127 B cell clones), RA patients (113 RF- and 60 RF+ B cell clones) and patients with primary Sjögren's syndrome (82 RF- and 47 RF+ B cell clones) by coculturing with anti-CD3-stimulated T helper cell clones. The cross-reactivity pattern of antibodies secreted by the B cell clones was determined by ELISA on a panel of antigens. The molecular structure of the IgM heavy chains was characterized by VH family-specific RT-PCR and sequencing. VH elements which correlated with RF specificity were identified. The responsiveness of B cells expressing these VH elements to T helper cell signals was compared in normal individuals and RA patients. RESULTS: The majority of RF+ B cells were monospecific when specificity was tested on five antigens. RF+ B cells expressed a significantly different repertoire of VH gene segments than RF- B cells. In particular, the VH3 gene segment V3-21 was not detected in B cell clones from normals but was the most frequent VH element in RF+ B cell clones from RA patients. Most of the V3-21 sequences were in germline configuration. The correlation between RF specificity and V3-21 gene segment usage was maintained in patients with Sjögren's syndrome. V3-21 transcripts were present in peripheral blood B cells from normal individuals. VH3-21+ B cells from RA patients but not from normal donors were responsive to preactivated T helper cells. Stimulation with a bacterial superantigen could overcome the nonresponsiveness of V3-21+ B cells in normal donors and induce the secretion of RF. CONCLUSIONS: RF production is correlated with the usage of the V3-21 gene segment in two distinct RF+ diseases. In patients with these diseases, V3-21+ B cells secrete antibodies with RF activity in response to activated T helper cells. V3-21+ B cells remain in a state of nonresponsiveness in normal individuals that can be broken by superantigen stimulation. The germline configuration of VH3-21+ RF+ immunoglobulins in RA patients suggests that the loss of tolerance is not an antigen-driven process.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adderson E. E., Shackelford P. G., Quinn A., Carroll W. L. Restricted Ig H chain V gene usage in the human antibody response to Haemophilus influenzae type b capsular polysaccharide. J Immunol. 1991 Sep 1;147(5):1667–1674. [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Bazan F., Blanchard D., Brière F., Galizzi J. P., van Kooten C., Liu Y. J., Rousset F., Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Buluwela L., Albertson D. G., Sherrington P., Rabbitts P. H., Spurr N., Rabbitts T. H. The use of chromosomal translocations to study human immunoglobulin gene organization: mapping DH segments within 35 kb of the C mu gene and identification of a new DH locus. EMBO J. 1988 Jul;7(7):2003–2010. doi: 10.1002/j.1460-2075.1988.tb03039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burastero S. E., Casali P., Wilder R. L., Notkins A. L. Monoreactive high affinity and polyreactive low affinity rheumatoid factors are produced by CD5+ B cells from patients with rheumatoid arthritis. J Exp Med. 1988 Dec 1;168(6):1979–1992. doi: 10.1084/jem.168.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Børretzen M., Randen I., Zdárský E., Førre O., Natvig J. B., Thompson K. M. Control of autoantibody affinity by selection against amino acid replacements in the complementarity-determining regions. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12917–12921. doi: 10.1073/pnas.91.26.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D. A., Chen P. P., Fox R. I., Kipps T. J., Jirik F., Goldfien R. D., Silverman G., Radoux V., Fong S. Rheumatoid factor and immune networks. Annu Rev Immunol. 1987;5:109–126. doi: 10.1146/annurev.iy.05.040187.000545. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Chen P. P., Kipps T. J. New roles for rheumatoid factor. J Clin Invest. 1991 Feb;87(2):379–383. doi: 10.1172/JCI115007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali P., Burastero S. E., Nakamura M., Inghirami G., Notkins A. L. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 1987 Apr 3;236(4797):77–81. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- Cook G. P., Tomlinson I. M., Walter G., Riethman H., Carter N. P., Buluwela L., Winter G., Rabbitts T. H. A map of the human immunoglobulin VH locus completed by analysis of the telomeric region of chromosome 14q. Nat Genet. 1994 Jun;7(2):162–168. doi: 10.1038/ng0694-162. [DOI] [PubMed] [Google Scholar]
- Cooke M. P., Heath A. W., Shokat K. M., Zeng Y., Finkelman F. D., Linsley P. S., Howard M., Goodnow C. C. Immunoglobulin signal transduction guides the specificity of B cell-T cell interactions and is blocked in tolerant self-reactive B cells. J Exp Med. 1994 Feb 1;179(2):425–438. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulie P. G., Van Snick J. Rheumatoid factor (RF) production during anamnestic immune responses in the mouse. III. Activation of RF precursor cells is induced by their interaction with immune complexes and carrier-specific helper T cells. J Exp Med. 1985 Jan 1;161(1):88–97. doi: 10.1084/jem.161.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Q., Kannapell C. C., Gaskin F., Solomon A., Koopman W. J., Fu S. M. Human rheumatoid factors with restrictive specificity for rabbit immunoglobulin G: auto- and multi-reactivity, diverse VH gene segment usage and preferential usage of V lambda IIIb. J Exp Med. 1994 May 1;179(5):1445–1456. doi: 10.1084/jem.179.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Shimizu M., Yamasaki K., Kishimoto T. Rheumatoid factor secretion from human Leu-1+ B cells. Science. 1987 Apr 3;236(4797):81–83. doi: 10.1126/science.3105057. [DOI] [PubMed] [Google Scholar]
- He X. W., Goronzy J., Weyand C. Selective induction of rheumatoid factors by superantigens and human helper T cells. J Clin Invest. 1992 Feb;89(2):673–680. doi: 10.1172/JCI115634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Goronzy J. J., Weyand C. M. The repertoire of rheumatoid factor-producing B cells in normal subjects and patients with rheumatoid arthritis. Arthritis Rheum. 1993 Aug;36(8):1061–1069. doi: 10.1002/art.1780360806. [DOI] [PubMed] [Google Scholar]
- Hillson J. L., Oppliger I. R., Sasso E. H., Milner E. C., Wener M. H. Emerging human B cell repertoire. Influence of developmental stage and interindividual variation. J Immunol. 1992 Dec 1;149(11):3741–3752. [PubMed] [Google Scholar]
- Ichihara Y., Matsuoka H., Kurosawa Y. Organization of human immunoglobulin heavy chain diversity gene loci. EMBO J. 1988 Dec 20;7(13):4141–4150. doi: 10.1002/j.1460-2075.1988.tb03309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps T. J., Duffy S. F. Relationship of the CD5 B cell to human tonsillar lymphocytes that express autoantibody-associated cross-reactive idiotypes. J Clin Invest. 1991 Jun;87(6):2087–2096. doi: 10.1172/JCI115239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–793. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- Lee S. K., Bridges S. L., Jr, Kirkham P. M., Koopman W. J., Schroeder H. W., Jr Evidence of antigen receptor-influenced oligoclonal B lymphocyte expansion in the synovium of a patient with longstanding rheumatoid arthritis. J Clin Invest. 1994 Jan;93(1):361–370. doi: 10.1172/JCI116968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. K., Bridges S. L., Jr, Koopman W. J., Schroeder H. W., Jr The immunoglobulin kappa light chain repertoire expressed in the synovium of a patient with rheumatoid arthritis. Arthritis Rheum. 1992 Aug;35(8):905–913. doi: 10.1002/art.1780350809. [DOI] [PubMed] [Google Scholar]
- Lucas A. H. Expression of crossreactive idiotypes by human antibodies specific for the capsular polysaccharide of Hemophilus influenzae B. J Clin Invest. 1988 Feb;81(2):480–486. doi: 10.1172/JCI113345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda F., Shin E. K., Hirabayashi Y., Nagaoka H., Yoshida M. C., Zong S. Q., Honjo T. Organization of variable region segments of the human immunoglobulin heavy chain: duplication of the D5 cluster within the locus and interchromosomal translocation of variable region segments. EMBO J. 1990 Aug;9(8):2501–2506. doi: 10.1002/j.1460-2075.1990.tb07429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda F., Shin E. K., Nagaoka H., Matsumura R., Haino M., Fukita Y., Taka-ishi S., Imai T., Riley J. H., Anand R. Structure and physical map of 64 variable segments in the 3'0.8-megabase region of the human immunoglobulin heavy-chain locus. Nat Genet. 1993 Jan;3(1):88–94. doi: 10.1038/ng0193-88. [DOI] [PubMed] [Google Scholar]
- Metlay J. P., Puré E., Steinman R. M. Control of the immune response at the level of antigen-presenting cells: a comparison of the function of dendritic cells and B lymphocytes. Adv Immunol. 1989;47:45–116. doi: 10.1016/s0065-2776(08)60662-8. [DOI] [PubMed] [Google Scholar]
- Olee T., Lu E. W., Huang D. F., Soto-Gil R. W., Deftos M., Kozin F., Carson D. A., Chen P. P. Genetic analysis of self-associating immunoglobulin G rheumatoid factors from two rheumatoid synovia implicates an antigen-driven response. J Exp Med. 1992 Mar 1;175(3):831–842. doi: 10.1084/jem.175.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual V., Capra J. D. Human immunoglobulin heavy-chain variable region genes: organization, polymorphism, and expression. Adv Immunol. 1991;49:1–74. doi: 10.1016/s0065-2776(08)60774-9. [DOI] [PubMed] [Google Scholar]
- Randen I., Brown D., Thompson K. M., Hughes-Jones N., Pascual V., Victor K., Capra J. D., Førre O., Natvig J. B. Clonally related IgM rheumatoid factors undergo affinity maturation in the rheumatoid synovial tissue. J Immunol. 1992 May 15;148(10):3296–3301. [PubMed] [Google Scholar]
- Randen I., Thompson K. M., Pascual V., Victor K., Beale D., Coadwell J., Førre O., Capra J. D., Natvig J. B. Rheumatoid factor V genes from patients with rheumatoid arthritis are diverse and show evidence of an antigen-driven response. Immunol Rev. 1992 Aug;128:49–71. doi: 10.1111/j.1600-065x.1992.tb00832.x. [DOI] [PubMed] [Google Scholar]
- Roosnek E., Lanzavecchia A. Efficient and selective presentation of antigen-antibody complexes by rheumatoid factor B cells. J Exp Med. 1991 Feb 1;173(2):487–489. doi: 10.1084/jem.173.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasso E. H., Barber C. V., Nardella F. A., Yount W. J., Mannik M. Antigenic specificities of human monoclonal and polyclonal IgM rheumatoid factors. The C gamma 2-C gamma 3 interface region contains the major determinants. J Immunol. 1988 May 1;140(9):3098–3107. [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Wang J. Y. Preferential utilization of conserved immunoglobulin heavy chain variable gene segments during human fetal life. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6146–6150. doi: 10.1073/pnas.87.16.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman G. J. Human antibody responses to bacterial antigens: studies of a model conventional antigen and a proposed model B cell superantigen. Int Rev Immunol. 1992;9(1):57–78. doi: 10.3109/08830189209061783. [DOI] [PubMed] [Google Scholar]
- Sonntag D., Weingärtner B., Grützmann R. A member of a novel human DH gene family: DHFL16. Nucleic Acids Res. 1989 Feb 11;17(3):1267–1267. doi: 10.1093/nar/17.3.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoflet E. S., Koeberl D. D., Sarkar G., Sommer S. S. Genomic amplification with transcript sequencing. Science. 1988 Jan 29;239(4839):491–494. doi: 10.1126/science.3340835. [DOI] [PubMed] [Google Scholar]
- Thiele D. L., Lipsky P. E. The immunosuppressive activity of L-leucyl-L-leucine methyl ester: selective ablation of cytotoxic lymphocytes and monocytes. J Immunol. 1986 Feb 1;136(3):1038–1048. [PubMed] [Google Scholar]
- Tighe H., Chen P. P., Tucker R., Kipps T. J., Roudier J., Jirik F. R., Carson D. A. Function of B cells expressing a human immunoglobulin M rheumatoid factor autoantibody in transgenic mice. J Exp Med. 1993 Jan 1;177(1):109–118. doi: 10.1084/jem.177.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohma S., Lipsky P. E. Analysis of the mechanisms of T cell-dependent polyclonal activation of human B cells. Induction of human B cell responses by fixed activated T cells. J Immunol. 1991 Apr 15;146(8):2544–2552. [PubMed] [Google Scholar]
- Vitali C., Bombardieri S., Moutsopoulos H. M., Balestrieri G., Bencivelli W., Bernstein R. M., Bjerrum K. B., Braga S., Coll J., de Vita S. Preliminary criteria for the classification of Sjögren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993 Mar;36(3):340–347. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. C., Jr, KUNKEL H. G. Rheumatoid factor, complement, and conglutinin aberrations in patients with subacute bacterial endocarditis. J Clin Invest. 1962 Mar;41:666–675. doi: 10.1172/JCI104523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walser-Kuntz D. R., Weyand C. M., Weaver A. J., O'Fallon W. M., Goronzy J. J. Mechanisms underlying the formation of the T cell receptor repertoire in rheumatoid arthritis. Immunity. 1995 Jun;2(6):597–605. doi: 10.1016/1074-7613(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., McCarthy T. G., Goronzy J. J. Correlation between disease phenotype and genetic heterogeneity in rheumatoid arthritis. J Clin Invest. 1995 May;95(5):2120–2126. doi: 10.1172/JCI117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Brühl H., He X., Weyand C. M., Goronzy J. J. Selective activation of VH3A10+ rheumatoid factor producing B cells by staphylococcal enterotoxin D. Int Immunol. 1995 Mar;7(3):425–434. doi: 10.1093/intimm/7.3.425. [DOI] [PubMed] [Google Scholar]
- Youngblood K., Fruchter L., Ding G., Lopez J., Bonagura V., Davidson A. Rheumatoid factors from the peripheral blood of two patients with rheumatoid arthritis are genetically heterogeneous and somatically mutated. J Clin Invest. 1994 Feb;93(2):852–861. doi: 10.1172/JCI117040. [DOI] [PMC free article] [PubMed] [Google Scholar]