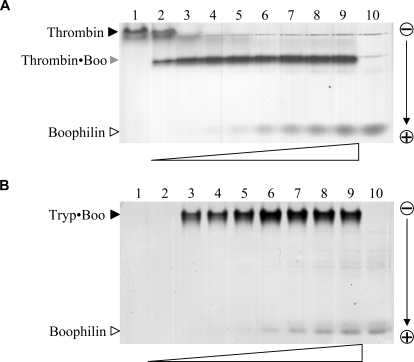
Figure 1. Boophilin forms stable stoichiometric complexes with free thrombin and trypsin.
(A) Five-hundred nanograms of free human α-thrombin were incubated with increasing amounts of the purified inhibitor (from ≈140 ng, lane 2, to ≈2.1 µg, lane 9), and samples were resolved in a 10% polyacrylamide gel. Lanes 1 and 10 contain 500 ng thrombin and 2.1 µg boophilin, respectively. Notice formation of a single species corresponding to the 1:1 thrombin·boophilin complex (Thrombin·Boo). (B) Detection of equimolar trypsin·boophilin complex. Five-hundred nanograms bovine trypsin were mixed with increasing amounts of purified boophilin (from ≈100 ng, lane 2, to 1.2 µg, lane 9), and samples were separated in a 10% polyacrylamide gel. Lanes 1 and 10 contain 500 ng trypsin and 1.2 µg boophilin, respectively; cationic trypsin does not migrate into the gel. The stoichiometric trypsin·boophilin complex is marked (Tryp·Boo).