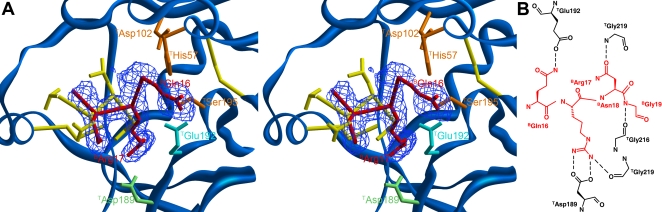
Figure 8. Boophilin inhibits thrombin in a non-canonical manner.
(A) Stereo close-up of the thrombin active centre (blue) showing the bound tetrapeptide BQ16–BR17–BN18–BG19 of boophilin (red), along with S1A–L1–N2–V3 of ornithodorin (yellow; notice that the N-terminal residue of the latter is an artifact introduced for cloning purposes). The final electron density for the thrombin·boophilin complex, contoured at 1σ, is displayed as a blue mesh. The catalytic triad of thrombin (TH57, TD102, TS195) is highlighted in orange and the side-chains of TD189 and TE192 are coloured pale green and cyan, respectively. Disulfide bonds are represented as yellow sticks. (B) Schematic representation of the thrombin-boophilin interactions at the enzyme's active site. Inhibitor residues are coloured red and hydrogen bonds are depicted as dashed lines.