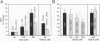Abstract
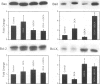
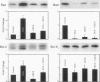
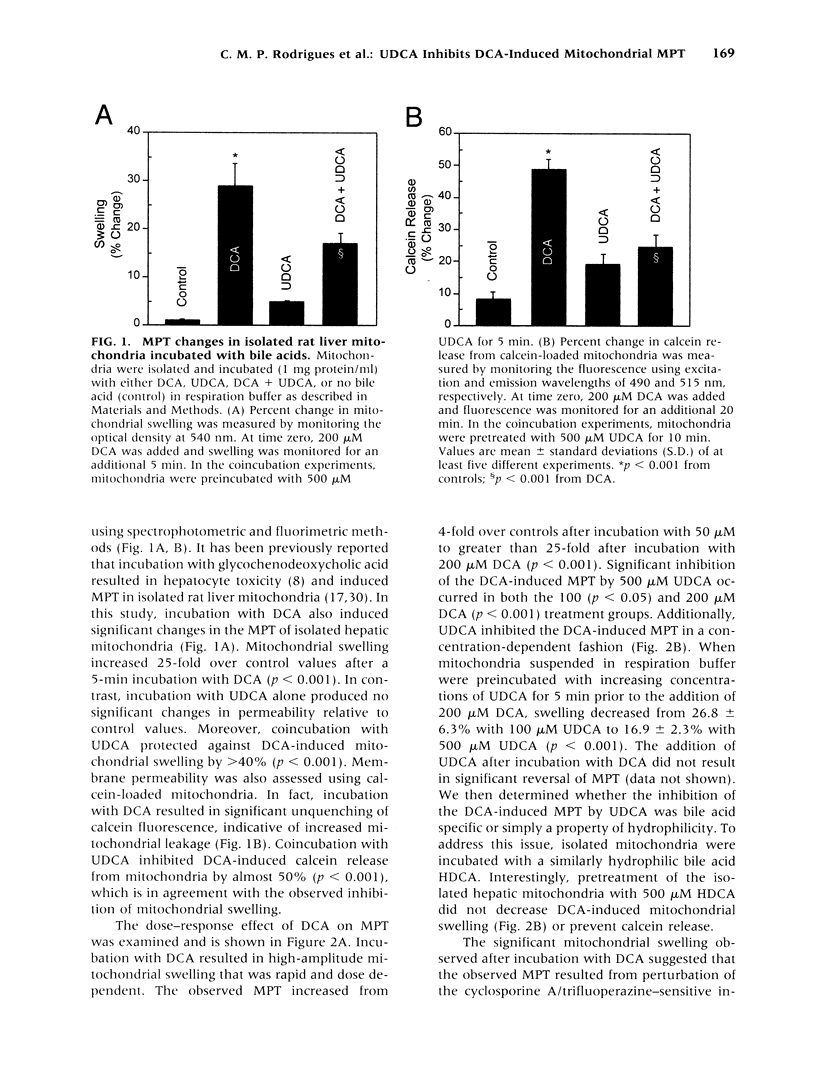
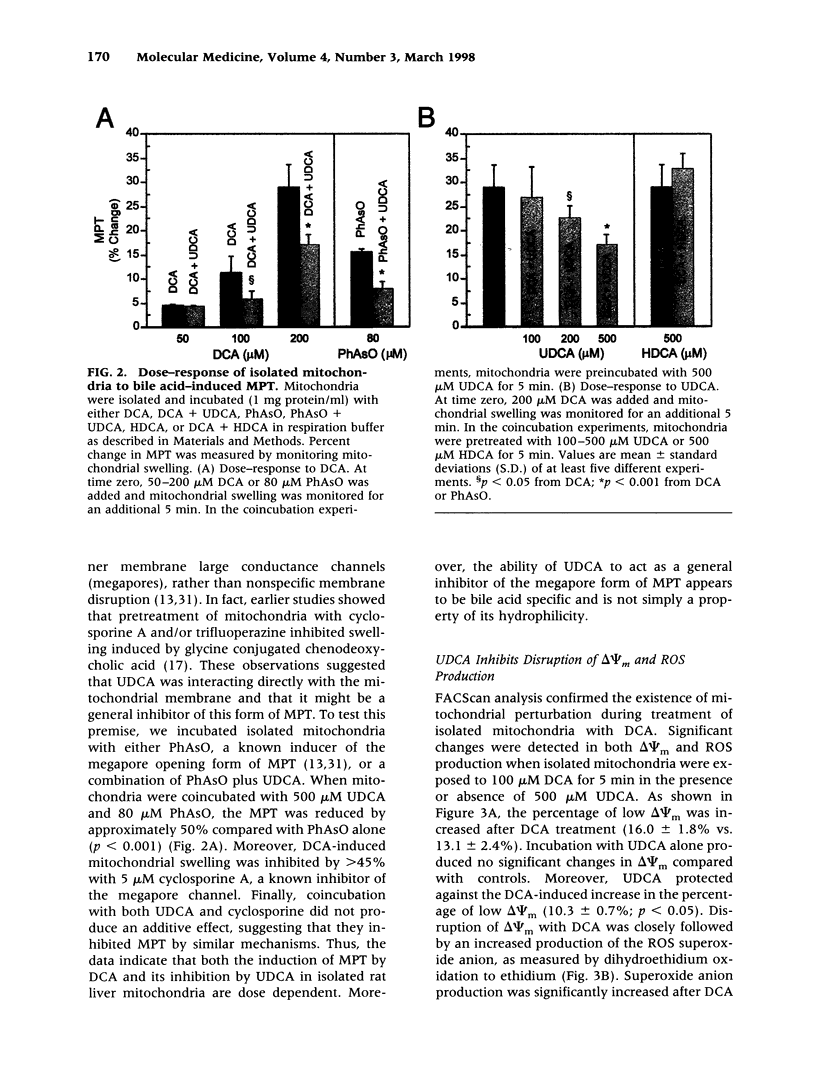
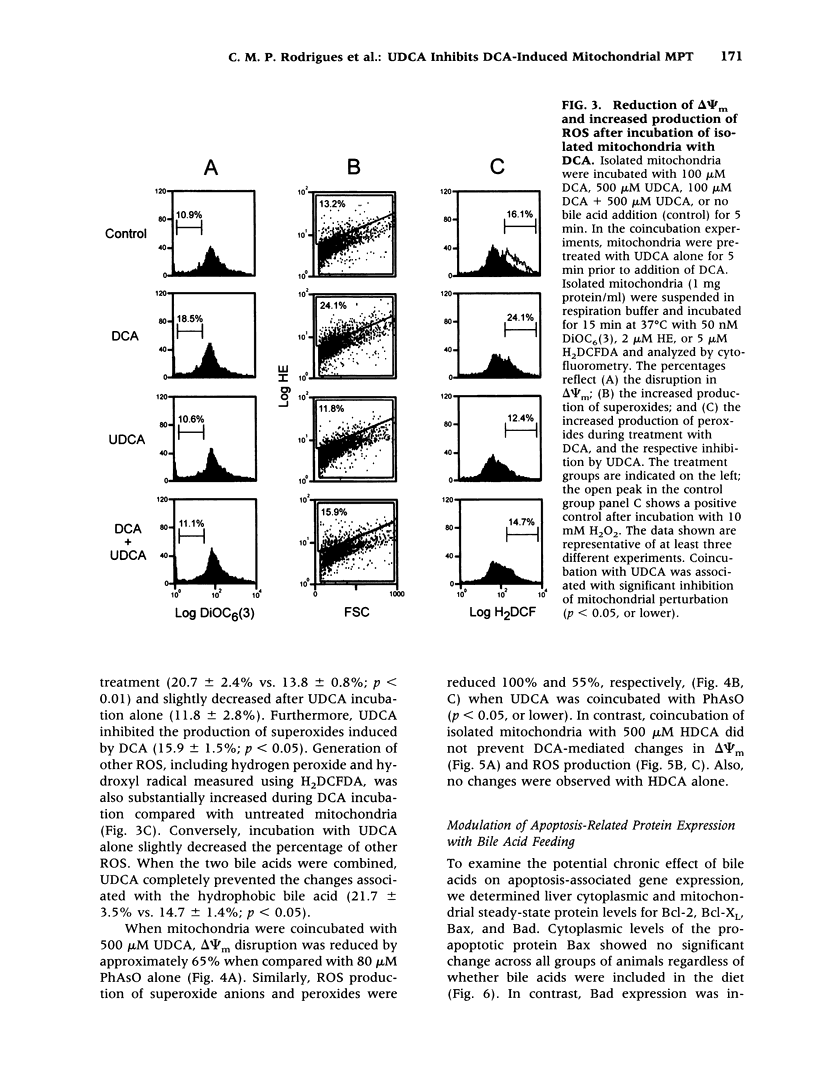
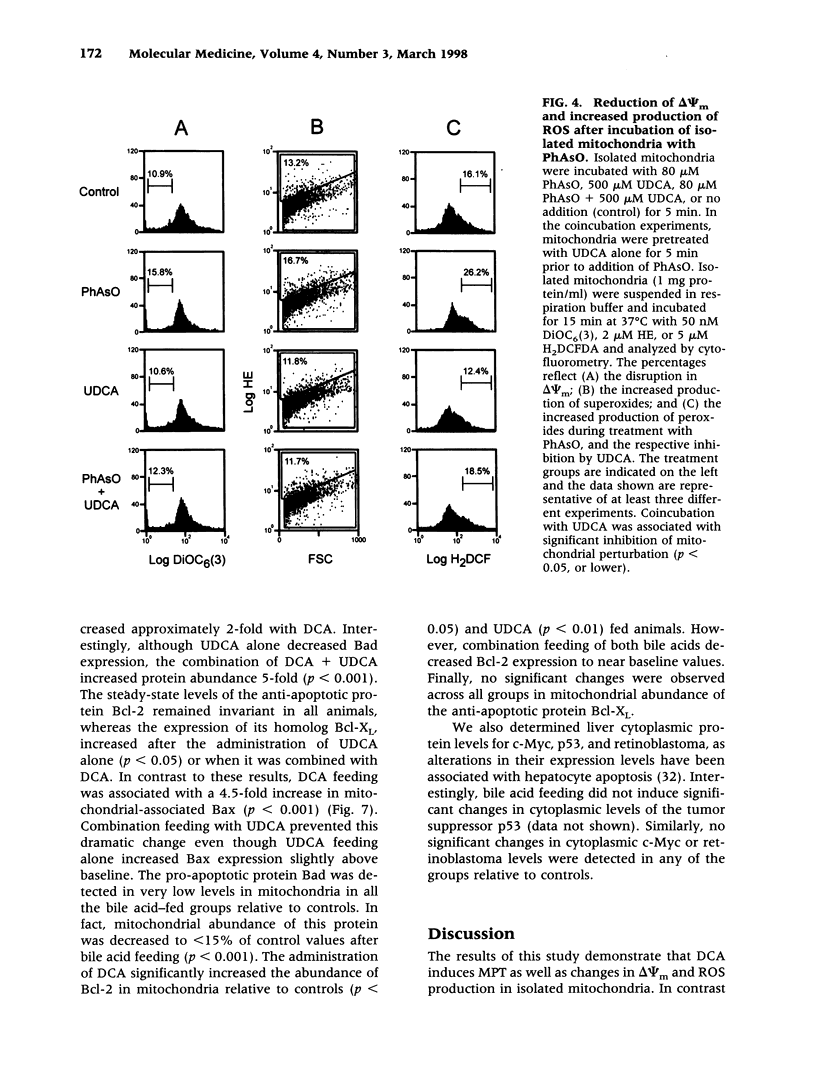
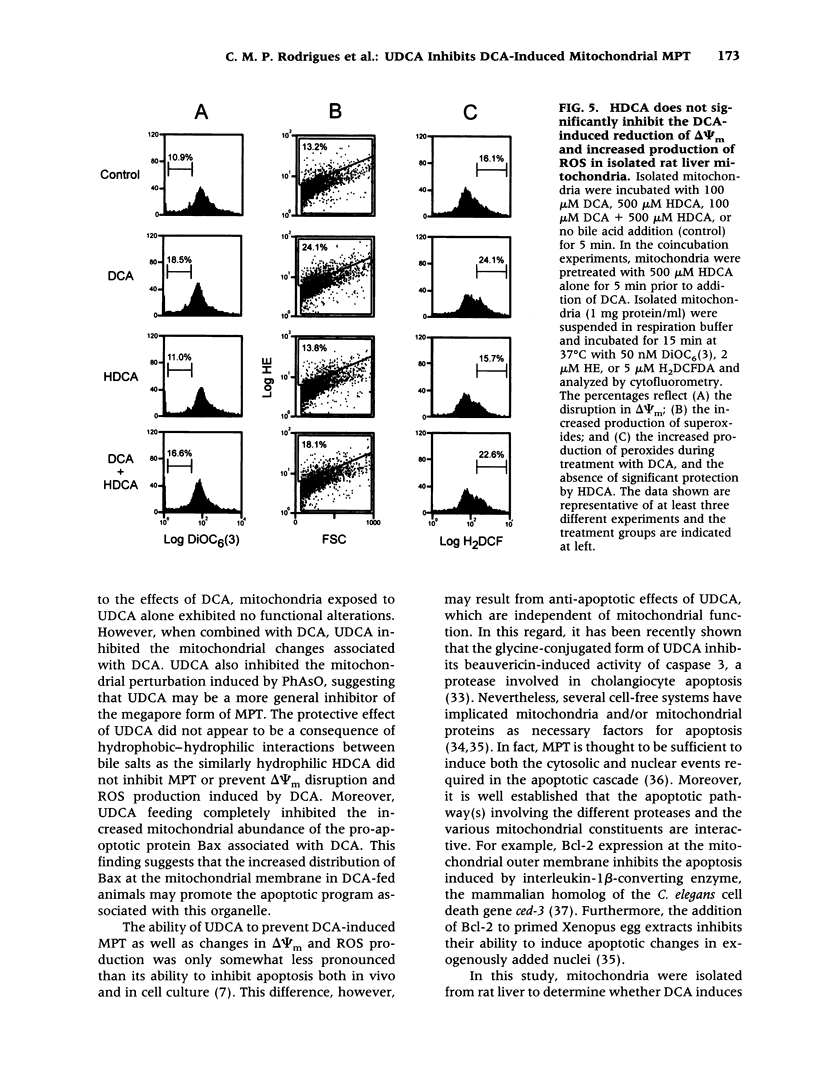
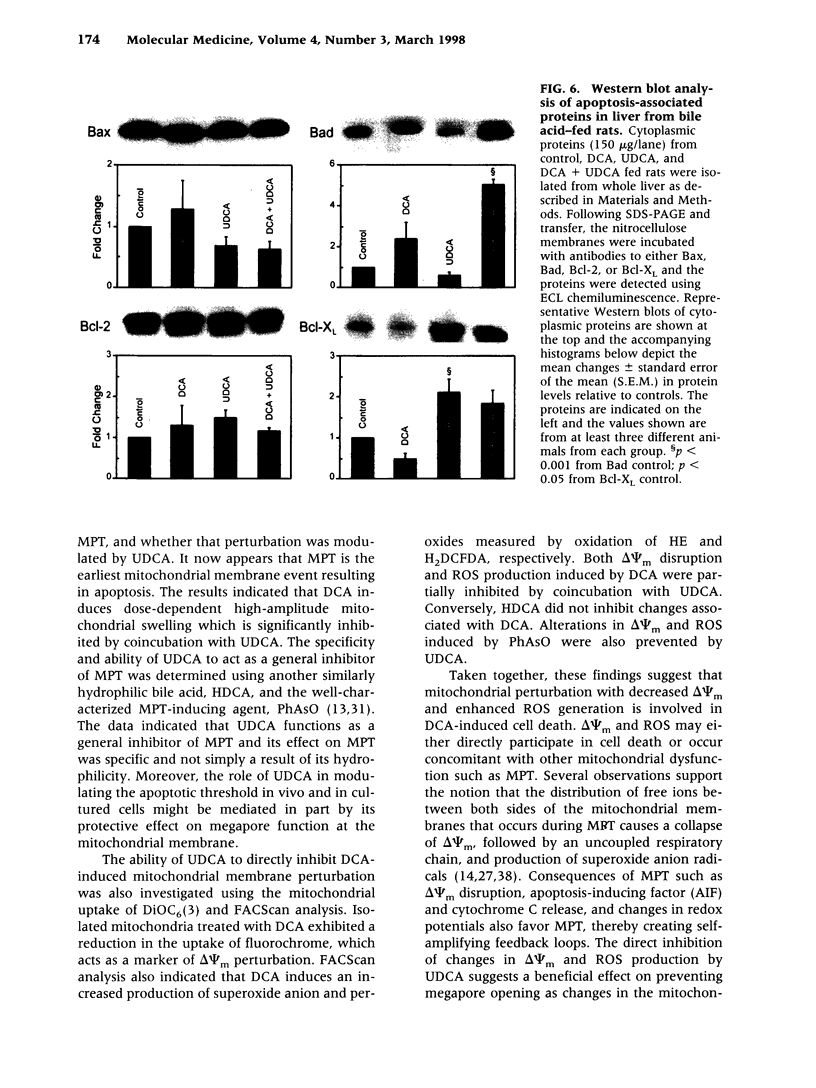
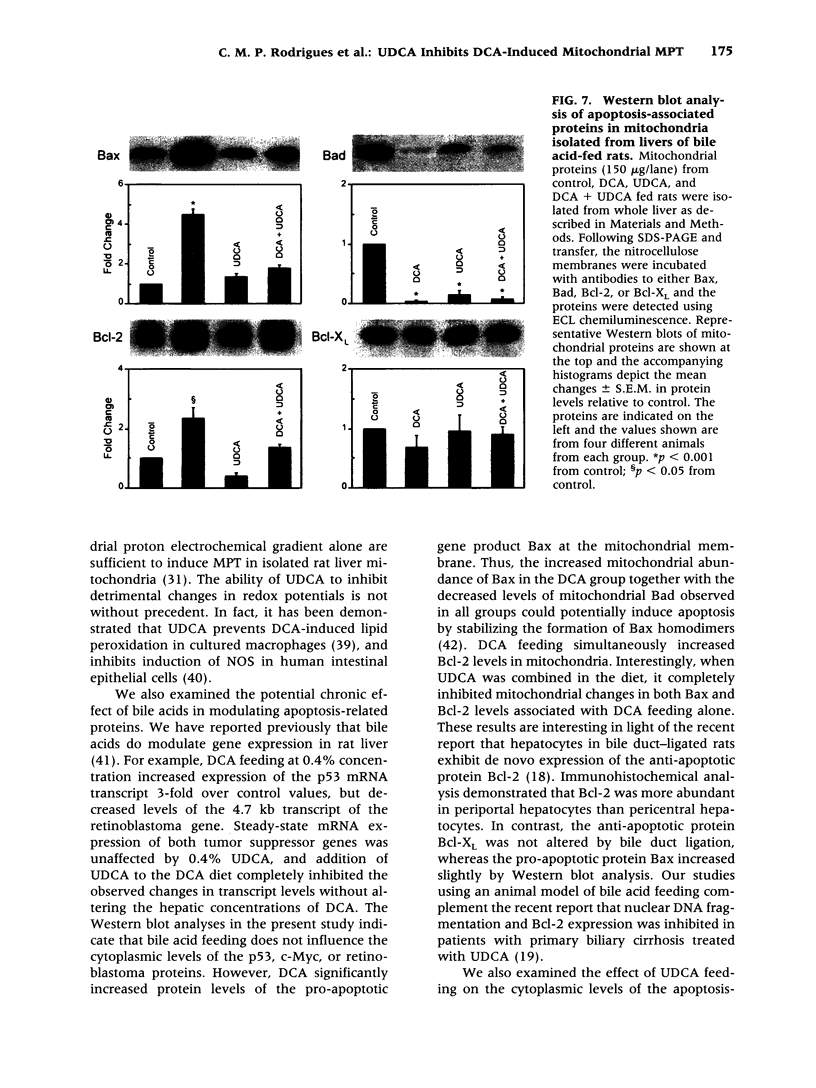
BACKGROUND: The hydrophilic bile salt ursodeoxycholate (UDCA) inhibits injury by hydrophobic bile acids and is used to treat cholestatic liver diseases. Interestingly, hepatocyte cell death from bile acid-induced toxicity occurs more frequently from apoptosis than from necrosis. However, both processes appear to involve the mitochondrial membrane permeability transition (MPT). In this study, we determined the inhibitory effect of UDCA on deoxycholic acid (DCA)-induced MPT in isolated mitochondria by measuring changes in transmembrane potential (delta psi m) and production of reactive oxygen species (ROS). In addition, we examined the expression of apoptosis-associated proteins in mitochondria isolated from livers of bile acid-fed animals. MATERIALS AND METHODS: Adult male rats were maintained on standard diet supplemented with DCA and/or UDCA for 10 days. Mitochondria were isolated from livers by sucrose/percoll gradient centrifugation and MPT was measured using spectrophotometric and fluorimetric assays. delta psi m and ROS generation were determined by FACScan analysis. Cytoplasmic and mitochondrial protein abundance were determined by Western blot analysis. RESULTS: DCA increased mitochondrial swelling 25-fold over controls (p < 0.001); UDCA reduced the swelling by > 40% (p < 0.001). Similarly, UDCA inhibited DCA-mediated release of calcein-loaded mitochondria by 50% (p < 0.001). delta psi m was significantly decreased in mitochondria incubated with DCA but not with UDCA. delta psi m disruption was followed closely by increased superoxide anion and peroxides production (p < 0.01). Coincubation of mitochondria with UDCA significantly inhibited the changes associated with DCA (p < 0.05). In vivo, DCA feeding was associated with a 4.5-fold increase in mitochondria-associated Bax protein levels (p < 0.001); combination feeding with UDCA almost totally inhibited this increase (p < 0.001). CONCLUSION: UDCA significantly reduces DCA-induced disruption of delta psi m, ROS production, and Bax protein abundance in mitochondria, suggesting both short- and long-term mechanisms in preventing MPT. The results suggest a possible role for UDCA as a therapeutic agent in the treatment of both hepatic and nonhepatic diseases associated with high levels of apoptosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaufay H., Amar-Costesec A., Feytmans E., Thinès-Sempoux D., Wibo M., Robbi M., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. I. Biochemical methods. J Cell Biol. 1974 Apr;61(1):188–200. doi: 10.1083/jcb.61.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by the proton electrochemical gradient. Evidence that the pore can be opened by membrane depolarization. J Biol Chem. 1992 May 5;267(13):8834–8839. [PubMed] [Google Scholar]
- Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993 Aug 27;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Botla R., Spivey J. R., Aguilar H., Bronk S. F., Gores G. J. Ursodeoxycholate (UDCA) inhibits the mitochondrial membrane permeability transition induced by glycochenodeoxycholate: a mechanism of UDCA cytoprotection. J Pharmacol Exp Ther. 1995 Feb;272(2):930–938. [PubMed] [Google Scholar]
- Carter W. O., Narayanan P. K., Robinson J. P. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukoc Biol. 1994 Feb;55(2):253–258. doi: 10.1002/jlb.55.2.253. [DOI] [PubMed] [Google Scholar]
- Cathcart R., Schwiers E., Ames B. N. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem. 1983 Oct 1;134(1):111–116. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- Chazouillères O., Poupon R., Capron J. P., Metman E. H., Dhumeaux D., Amouretti M., Couzigou P., Labayle D., Trinchet J. C. Ursodeoxycholic acid for primary sclerosing cholangitis. J Hepatol. 1990 Jul;11(1):120–123. doi: 10.1016/0168-8278(90)90281-u. [DOI] [PubMed] [Google Scholar]
- Heuman D. M., Mills A. S., McCall J., Hylemon P. B., Pandak W. M., Vlahcevic Z. R. Conjugates of ursodeoxycholate protect against cholestasis and hepatocellular necrosis caused by more hydrophobic bile salts. In vivo studies in the rat. Gastroenterology. 1991 Jan;100(1):203–211. doi: 10.1016/0016-5085(91)90602-h. [DOI] [PubMed] [Google Scholar]
- Imberti R., Nieminen A. L., Herman B., Lemasters J. J. Mitochondrial and glycolytic dysfunction in lethal injury to hepatocytes by t-butylhydroperoxide: protection by fructose, cyclosporin A and trifluoperazine. J Pharmacol Exp Ther. 1993 Apr;265(1):392–400. [PubMed] [Google Scholar]
- Invernizzi P., Salzman A. L., Szabó C., Ueta I., O'Connor M., Setchell K. D. Ursodeoxycholate inhibits induction of NOS in human intestinal epithelial cells and in vivo. Am J Physiol. 1997 Jul;273(1 Pt 1):G131–G138. doi: 10.1152/ajpgi.1997.273.1.G131. [DOI] [PubMed] [Google Scholar]
- Kantrow S. P., Piantadosi C. A. Release of cytochrome c from liver mitochondria during permeability transition. Biochem Biophys Res Commun. 1997 Mar 27;232(3):669–671. doi: 10.1006/bbrc.1997.6353. [DOI] [PubMed] [Google Scholar]
- Kluck R. M., Bossy-Wetzel E., Green D. R., Newmeyer D. D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997 Feb 21;275(5303):1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Koga H., Sakisaka S., Ohishi M., Sata M., Tanikawa K. Nuclear DNA fragmentation and expression of Bcl-2 in primary biliary cirrhosis. Hepatology. 1997 May;25(5):1077–1084. doi: 10.1002/hep.510250505. [DOI] [PubMed] [Google Scholar]
- Kren B. T., Kumar N. M., Wang S. Q., Gilula N. B., Steer C. J. Differential regulation of multiple gap junction transcripts and proteins during rat liver regeneration. J Cell Biol. 1993 Nov;123(3):707–718. doi: 10.1083/jcb.123.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kren B. T., Rodrigues C. M., Setchell K. D., Steer C. J. Posttranscriptional regulation of mRNA levels in rat liver associated with deoxycholic acid feeding. Am J Physiol. 1995 Dec;269(6 Pt 1):G961–G973. doi: 10.1152/ajpgi.1995.269.6.G961. [DOI] [PubMed] [Google Scholar]
- Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997 Jun;3(6):614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- Kroemer G., Zamzami N., Susin S. A. Mitochondrial control of apoptosis. Immunol Today. 1997 Jan;18(1):44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- Krähenbühl S., Stucki J., Reichen J. Reduced activity of the electron transport chain in liver mitochondria isolated from rats with secondary biliary cirrhosis. Hepatology. 1992 Jun;15(6):1160–1166. doi: 10.1002/hep.1840150630. [DOI] [PubMed] [Google Scholar]
- Krähenbühl S., Talos C., Fischer S., Reichen J. Toxicity of bile acids on the electron transport chain of isolated rat liver mitochondria. Hepatology. 1994 Feb;19(2):471–479. doi: 10.1002/hep.1840190228. [DOI] [PubMed] [Google Scholar]
- Kurosawa H., Que F. G., Roberts L. R., Fesmier P. J., Gores G. J. Hepatocytes in the bile duct-ligated rat express Bcl-2. Am J Physiol. 1997 Jun;272(6 Pt 1):G1587–G1593. doi: 10.1152/ajpgi.1997.272.6.G1587. [DOI] [PubMed] [Google Scholar]
- LaRusso N. F., Fowler S. Coordinate secretion of acid hydrolases in rat bile. J Clin Invest. 1979 Oct;64(4):948–954. doi: 10.1172/JCI109561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubuncic P., Fuhrman B., Oiknine J., Aviram M., Bomzon A. Effect of deoxycholic acid and ursodeoxycholic acid on lipid peroxidation in cultured macrophages. Gut. 1996 Sep;39(3):475–478. doi: 10.1136/gut.39.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M., Zhu H., Rotello R., Hartwieg E. A., Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993 Nov 19;75(4):653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Newmeyer D. D., Farschon D. M., Reed J. C. Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell. 1994 Oct 21;79(2):353–364. doi: 10.1016/0092-8674(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Pastorino J. G., Snyder J. W., Serroni A., Hoek J. B., Farber J. L. Cyclosporin and carnitine prevent the anoxic death of cultured hepatocytes by inhibiting the mitochondrial permeability transition. J Biol Chem. 1993 Jul 5;268(19):13791–13798. [PubMed] [Google Scholar]
- Podda M., Ghezzi C., Battezzati P. M., Crosignani A., Zuin M., Roda A. Effects of ursodeoxycholic acid and taurine on serum liver enzymes and bile acids in chronic hepatitis. Gastroenterology. 1990 Apr;98(4):1044–1050. doi: 10.1016/0016-5085(90)90032-v. [DOI] [PubMed] [Google Scholar]
- Poupon R. E., Poupon R., Balkau B. Ursodiol for the long-term treatment of primary biliary cirrhosis. The UDCA-PBC Study Group. N Engl J Med. 1994 May 12;330(19):1342–1347. doi: 10.1056/NEJM199405123301903. [DOI] [PubMed] [Google Scholar]
- Quist R. G., Ton-Nu H. T., Lillienau J., Hofmann A. F., Barrett K. E. Activation of mast cells by bile acids. Gastroenterology. 1991 Aug;101(2):446–456. doi: 10.1016/0016-5085(91)90024-f. [DOI] [PubMed] [Google Scholar]
- Schaffner F., Bacchin P. G., Hutterer F., Scharnbeck H. H., Sarkozi L. L., Denk H., Popper H. Mechanism of cholestasis. 4. Structural and biochemical changes in the liver and serum in rats after bile duct ligation. Gastroenterology. 1971 May;60(5):888–897. [PubMed] [Google Scholar]
- Schmucker D. L., Ohta M., Kanai S., Sato Y., Kitani K. Hepatic injury induced by bile salts: correlation between biochemical and morphological events. Hepatology. 1990 Nov;12(5):1216–1221. doi: 10.1002/hep.1840120523. [DOI] [PubMed] [Google Scholar]
- Sokol R. J., Devereaux M., Khandwala R., O'Brien K. Evidence for involvement of oxygen free radicals in bile acid toxicity to isolated rat hepatocytes. Hepatology. 1993 May;17(5):869–881. [PubMed] [Google Scholar]
- Sokol R. J., Devereaux M., Mierau G. W., Hambidge K. M., Shikes R. H. Oxidant injury to hepatic mitochondrial lipids in rats with dietary copper overload. Modification by vitamin E deficiency. Gastroenterology. 1990 Oct;99(4):1061–1071. doi: 10.1016/0016-5085(90)90627-d. [DOI] [PubMed] [Google Scholar]
- Spivey J. R., Bronk S. F., Gores G. J. Glycochenodeoxycholate-induced lethal hepatocellular injury in rat hepatocytes. Role of ATP depletion and cytosolic free calcium. J Clin Invest. 1993 Jul;92(1):17–24. doi: 10.1172/JCI116546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin S. A., Zamzami N., Castedo M., Hirsch T., Marchetti P., Macho A., Daugas E., Geuskens M., Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996 Oct 1;184(4):1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trembley J. H., Ebbert J. O., Kren B. T., Steer C. J. Differential regulation of cyclin B1 RNA and protein expression during hepatocyte growth in vivo. Cell Growth Differ. 1996 Jul;7(7):903–916. [PubMed] [Google Scholar]
- Vander Heiden M. G., Chandel N. S., Williamson E. K., Schumacker P. T., Thompson C. B. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997 Nov 28;91(5):627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- Vayssiere J. L., Petit P. X., Risler Y., Mignotte B. Commitment to apoptosis is associated with changes in mitochondrial biogenesis and activity in cell lines conditionally immortalized with simian virus 40. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11752–11756. doi: 10.1073/pnas.91.24.11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walajtys-Rhode E., Zapatero J., Moehren G., Hoek J. B. The role of the matrix calcium level in the enhancement of mitochondrial pyruvate carboxylation by glucagon pretreatment. J Biol Chem. 1992 Jan 5;267(1):370–379. [PubMed] [Google Scholar]
- Xiang J., Chao D. T., Korsmeyer S. J. BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E., Zha J., Jockel J., Boise L. H., Thompson C. B., Korsmeyer S. J. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995 Jan 27;80(2):285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- Yang J., Liu X., Bhalla K., Kim C. N., Ibrado A. M., Cai J., Peng T. I., Jones D. P., Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997 Feb 21;275(5303):1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Zamzami N., Marchetti P., Castedo M., Decaudin D., Macho A., Hirsch T., Susin S. A., Petit P. X., Mignotte B., Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med. 1995 Aug 1;182(2):367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamzami N., Marchetti P., Castedo M., Zanin C., Vayssière J. L., Petit P. X., Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med. 1995 May 1;181(5):1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamzami N., Susin S. A., Marchetti P., Hirsch T., Gómez-Monterrey I., Castedo M., Kroemer G. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996 Apr 1;183(4):1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]