Abstract
Presenilins 1 (PS1) and 2 (PS2) are multispanning transmembrane proteins associated with familial Alzheimer disease (FAD). They are developmentally regulated, being expressed at highest levels during neuronal differentiation and are sustained at a lower level throughout life. We investigated the distribution and metabolism of endogenous murine PS1 as well as human wild-type (wtPS1) and the familial AD Met146Leu (M146L) mutant presenilins in dissociated cultures of hippocampal neurons derived from control and transgenic mice. We found that the PS1 endoproteolytic fragments and, to a lesser extent, the full-length protein, were expressed as early as day 3 post-plating. Both species increased until the cells were fully differentiated at day 12. Confocal microscopy revealed that presenilin is present in the Golgi and endoplasmic reticulum and, as in punctate, vesicle-like structures within developing neurites and growth cones. Using a human-specific PS1 antibody, we were able to independently examine the distribution of the transgenic protein which, although similar to the endogenous, showed some unique qualities. These included (i) some heterogeneity in the proteolytic fragments of human PS1; (ii) significantly reduced levels of full-length human PS1, possibly as a result of preferential processing; and (iii) a more discrete intracellular distribution of human PS1. Colocalization with organelle-specific proteins revealed that PS1 was located in a diffuse staining pattern in the MAP2-positive dendrites and in a punctate manner in GAP43-positive axons. PS1 showed considerable overlap with GAP43, particularly at the growth cones. Similar patterns of PS1 distribution were detected in cultures derived from transgenic animals expressing human wild-type or mutant presenilins. The studies demonstrate that mutant presenilins are not grossly different in their processing or distribution within cultured neurons, which may represent more physiological models as compared to transfection systems. Our data also suggest that the molecular pathology associated with PS1 mutations results from subtle alterations in presenilin function, which can be further investigated using these transgenic neuronal cell culture models.
Full text
PDF


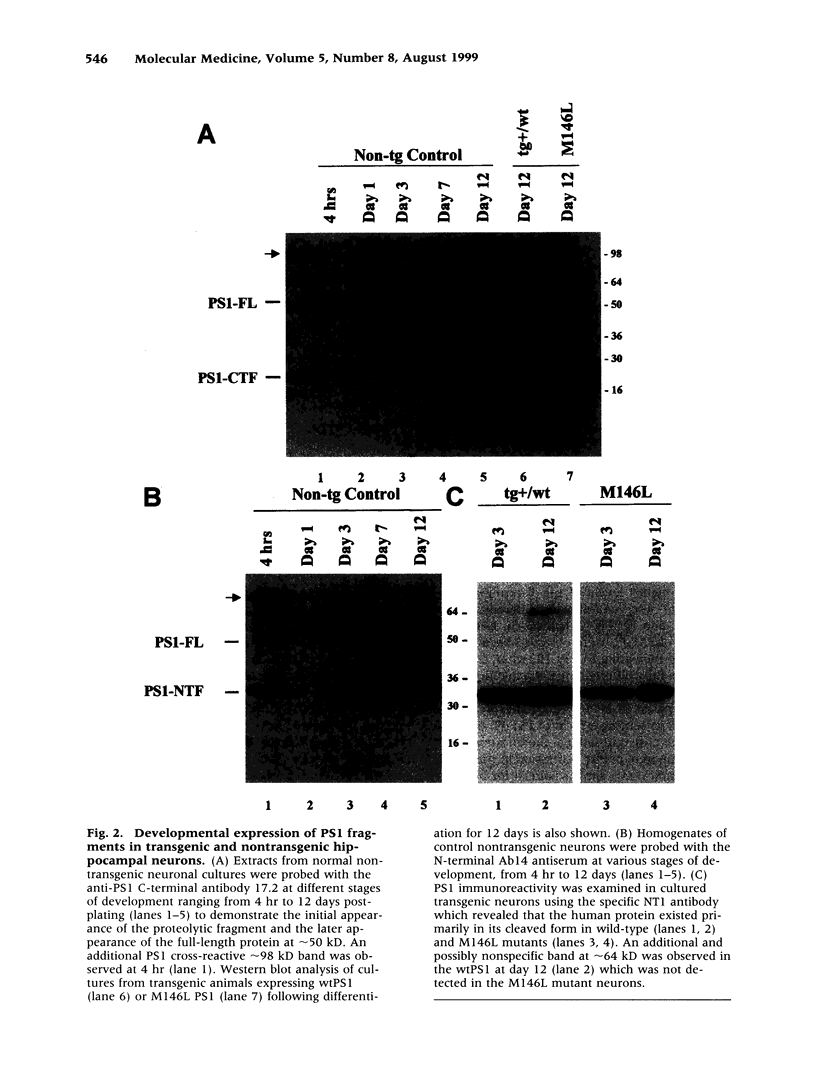
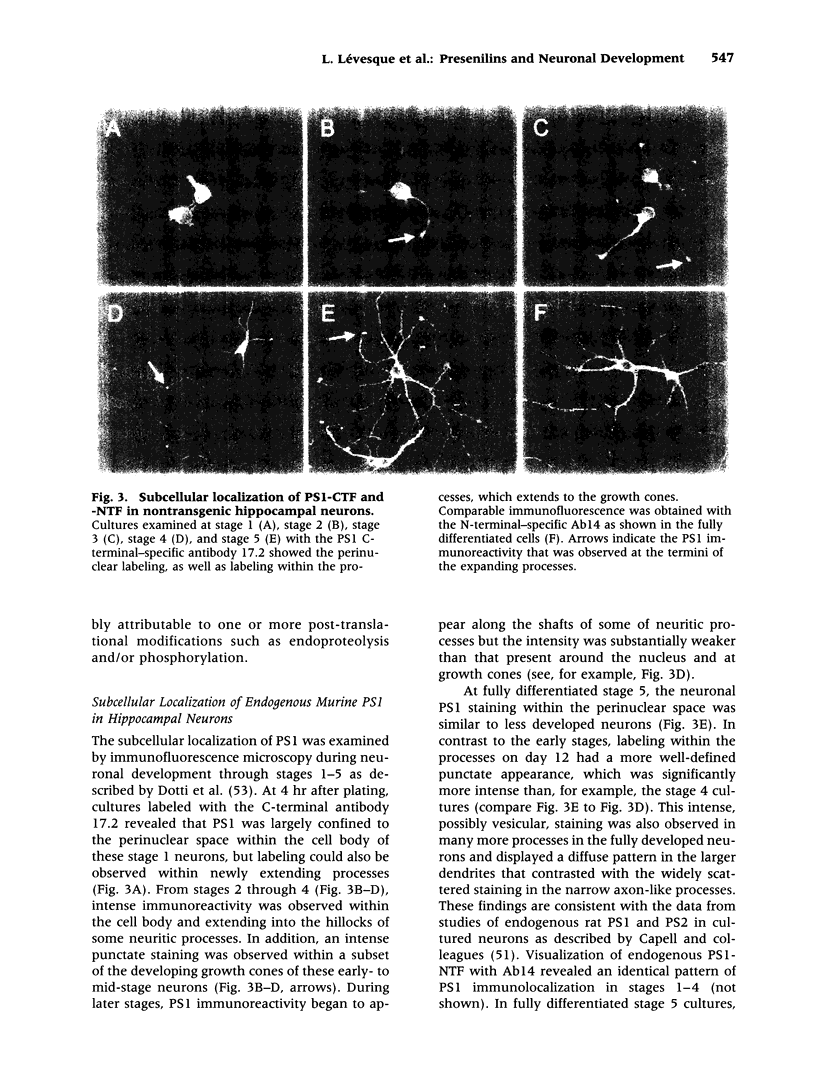

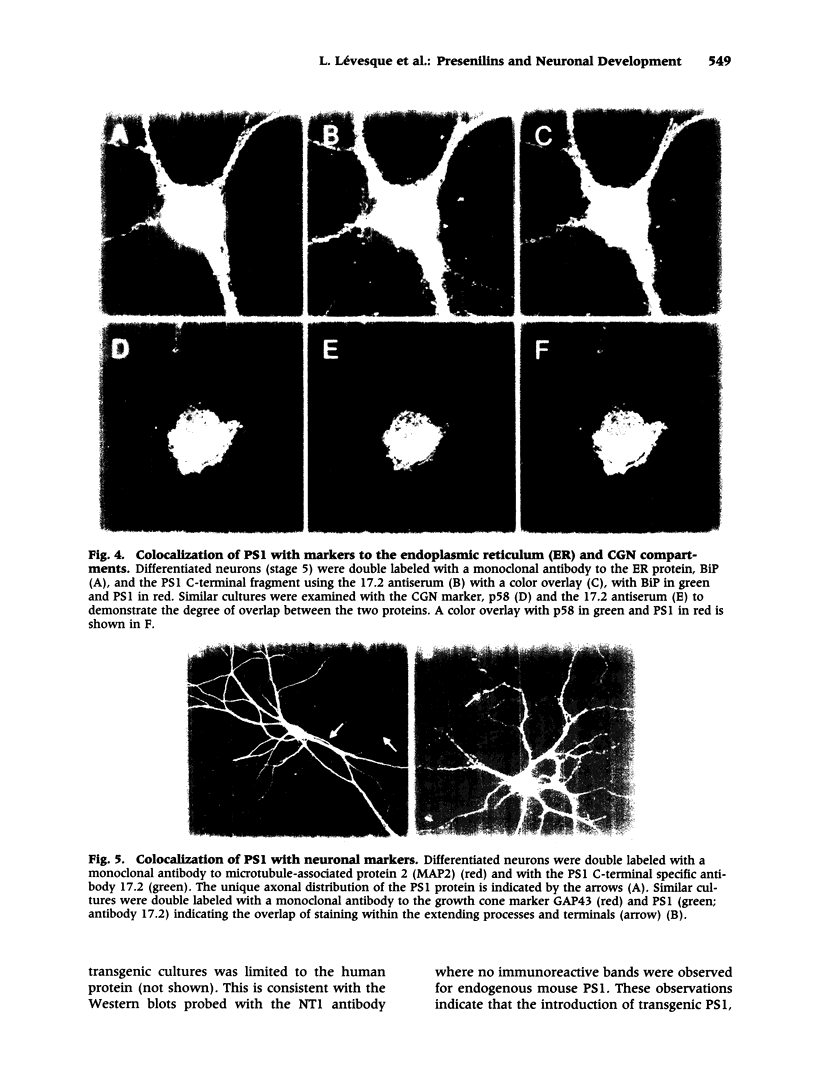
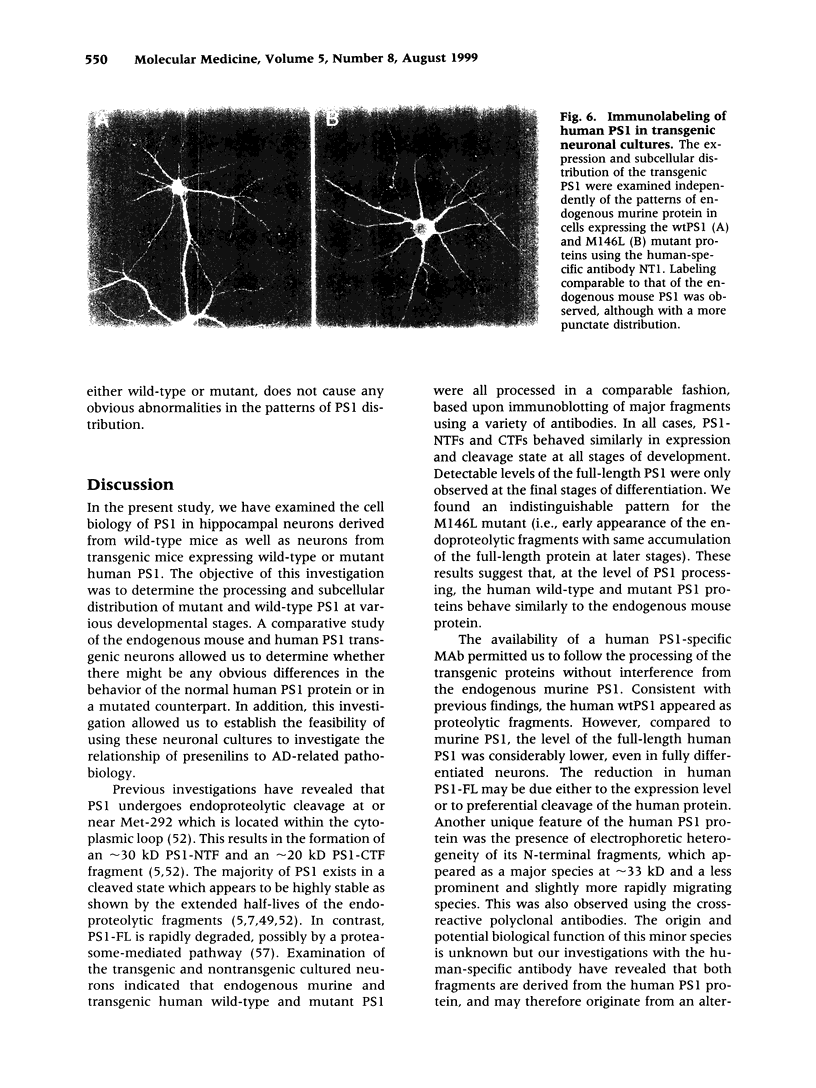




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumeister R., Leimer U., Zweckbronner I., Jakubek C., Grünberg J., Haass C. Human presenilin-1, but not familial Alzheimer's disease (FAD) mutants, facilitate Caenorhabditis elegans Notch signalling independently of proteolytic processing. Genes Funct. 1997 Apr;1(2):149–159. doi: 10.1046/j.1365-4624.1997.00012.x. [DOI] [PubMed] [Google Scholar]
- Beher D., Elle C., Underwood J., Davis J. B., Ward R., Karran E., Masters C. L., Beyreuther K., Multhaup G. Proteolytic fragments of Alzheimer's disease-associated presenilin 1 are present in synaptic organelles and growth cone membranes of rat brain. J Neurochem. 1999 Apr;72(4):1564–1573. doi: 10.1046/j.1471-4159.1999.721564.x. [DOI] [PubMed] [Google Scholar]
- Berezovska O., Xia M. Q., Page K., Wasco W., Tanzi R. E., Hyman B. T. Developmental regulation of presenilin mRNA expression parallels notch expression. J Neuropathol Exp Neurol. 1997 Jan;56(1):40–44. doi: 10.1097/00005072-199701000-00004. [DOI] [PubMed] [Google Scholar]
- Borchelt D. R., Ratovitski T., van Lare J., Lee M. K., Gonzales V., Jenkins N. A., Copeland N. G., Price D. L., Sisodia S. S. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997 Oct;19(4):939–945. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- Borchelt D. R., Thinakaran G., Eckman C. B., Lee M. K., Davenport F., Ratovitsky T., Prada C. M., Kim G., Seekins S., Yager D. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996 Nov;17(5):1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- Capell A., Saffrich R., Olivo J. C., Meyn L., Walter J., Grünberg J., Mathews P., Nixon R., Dotti C., Haass C. Cellular expression and proteolytic processing of presenilin proteins is developmentally regulated during neuronal differentiation. J Neurochem. 1997 Dec;69(6):2432–2440. doi: 10.1046/j.1471-4159.1997.69062432.x. [DOI] [PubMed] [Google Scholar]
- Citron M., Westaway D., Xia W., Carlson G., Diehl T., Levesque G., Johnson-Wood K., Lee M., Seubert P., Davis A. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med. 1997 Jan;3(1):67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- Conlon R. A., Reaume A. G., Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995 May;121(5):1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- Cook D. G., Sung J. C., Golde T. E., Felsenstein K. M., Wojczyk B. S., Tanzi R. E., Trojanowski J. Q., Lee V. M., Doms R. W. Expression and analysis of presenilin 1 in a human neuronal system: localization in cell bodies and dendrites. Proc Natl Acad Sci U S A. 1996 Aug 20;93(17):9223–9228. doi: 10.1073/pnas.93.17.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A. M., Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- Cribbs D. H., Chen L. S., Bende S. M., LaFerla F. M. Widespread neuronal expression of the presenilin-1 early-onset Alzheimer's disease gene in the murine brain. Am J Pathol. 1996 Jun;148(6):1797–1806. [PMC free article] [PubMed] [Google Scholar]
- Culvenor J. G., Maher F., Evin G., Malchiodi-Albedi F., Cappai R., Underwood J. R., Davis J. B., Karran E. H., Roberts G. W., Beyreuther K. Alzheimer's disease-associated presenilin 1 in neuronal cells: evidence for localization to the endoplasmic reticulum-Golgi intermediate compartment. J Neurosci Res. 1997 Sep 15;49(6):719–731. doi: 10.1002/(SICI)1097-4547(19970915)49:6<719::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Annaert W., Cupers P., Saftig P., Craessaerts K., Mumm J. S., Schroeter E. H., Schrijvers V., Wolfe M. S., Ray W. J. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999 Apr 8;398(6727):518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Beullens M., Contreras B., Levesque L., Craessaerts K., Cordell B., Moechars D., Bollen M., Fraser P., George-Hyslop P. S. Phosphorylation, subcellular localization, and membrane orientation of the Alzheimer's disease-associated presenilins. J Biol Chem. 1997 Feb 7;272(6):3590–3598. doi: 10.1074/jbc.272.6.3590. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Saftig P., Craessaerts K., Vanderstichele H., Guhde G., Annaert W., Von Figura K., Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998 Jan 22;391(6665):387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- Doan A., Thinakaran G., Borchelt D. R., Slunt H. H., Ratovitsky T., Podlisny M., Selkoe D. J., Seeger M., Gandy S. E., Price D. L. Protein topology of presenilin 1. Neuron. 1996 Nov;17(5):1023–1030. doi: 10.1016/s0896-6273(00)80232-9. [DOI] [PubMed] [Google Scholar]
- Dotti C. G., Sullivan C. A., Banker G. A. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988 Apr;8(4):1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K., Eckman C., Zehr C., Yu X., Prada C. M., Perez-tur J., Hutton M., Buee L., Harigaya Y., Yager D. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996 Oct 24;383(6602):710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- Elder G. A., Tezapsidis N., Carter J., Shioi J., Bouras C., Li H. C., Johnston J. M., Efthimiopoulos S., Friedrich V. L., Jr, Robakis N. K. Identification and neuron specific expression of the S182/presenilin I protein in human and rodent brains. J Neurosci Res. 1996 Aug 1;45(3):308–320. doi: 10.1002/(SICI)1097-4547(19960801)45:3<308::AID-JNR13>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Fraser P. E., Levesque G., Yu G., Mills L. R., Thirlwell J., Frantseva M., Gandy S. E., Seeger M., Carlen P. L., St George-Hyslop P. Presenilin 1 is actively degraded by the 26S proteasome. Neurobiol Aging. 1998 Jan-Feb;19(1 Suppl):S19–S21. doi: 10.1016/s0197-4580(98)00029-3. [DOI] [PubMed] [Google Scholar]
- Guo Q., Furukawa K., Sopher B. L., Pham D. G., Xie J., Robinson N., Martin G. M., Mattson M. P. Alzheimer's PS-1 mutation perturbs calcium homeostasis and sensitizes PC12 cells to death induced by amyloid beta-peptide. Neuroreport. 1996 Dec 20;8(1):379–383. doi: 10.1097/00001756-199612200-00074. [DOI] [PubMed] [Google Scholar]
- Hartmann H., Busciglio J., Baumann K. H., Staufenbiel M., Yankner B. A. Developmental regulation of presenilin-1 processing in the brain suggests a role in neuronal differentiation. J Biol Chem. 1997 Jun 6;272(23):14505–14508. doi: 10.1074/jbc.272.23.14505. [DOI] [PubMed] [Google Scholar]
- Johnston J. A., Froelich S., Lannfelt L., Cowburn R. F. Quantification of presenilin-1 mRNA in Alzheimer's disease brains. FEBS Lett. 1996 Oct 7;394(3):279–284. doi: 10.1016/0014-5793(96)00969-6. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Wegiel J., Sapienza V., Chen J., Hong H., Wisniewski H. M. Immunoreactivity of presenilin-1 in human, rat and mouse brain. Brain Res. 1997 May 16;757(1):159–163. doi: 10.1016/s0006-8993(97)00243-6. [DOI] [PubMed] [Google Scholar]
- Kovacs D. M., Fausett H. J., Page K. J., Kim T. W., Moir R. D., Merriam D. E., Hollister R. D., Hallmark O. G., Mancini R., Felsenstein K. M. Alzheimer-associated presenilins 1 and 2: neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nat Med. 1996 Feb;2(2):224–229. doi: 10.1038/nm0296-224. [DOI] [PubMed] [Google Scholar]
- Krijnse-Locker J., Parton R. G., Fuller S. D., Griffiths G., Dotti C. G. The organization of the endoplasmic reticulum and the intermediate compartment in cultured rat hippocampal neurons. Mol Biol Cell. 1995 Oct;6(10):1315–1332. doi: 10.1091/mbc.6.10.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lah J. J., Heilman C. J., Nash N. R., Rees H. D., Yi H., Counts S. E., Levey A. I. Light and electron microscopic localization of presenilin-1 in primate brain. J Neurosci. 1997 Mar 15;17(6):1971–1980. doi: 10.1523/JNEUROSCI.17-06-01971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. K., Slunt H. H., Martin L. J., Thinakaran G., Kim G., Gandy S. E., Seeger M., Koo E., Price D. L., Sisodia S. S. Expression of presenilin 1 and 2 (PS1 and PS2) in human and murine tissues. J Neurosci. 1996 Dec 1;16(23):7513–7525. doi: 10.1523/JNEUROSCI.16-23-07513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere C. A., Lopera F., Kosik K. S., Lendon C. L., Ossa J., Saido T. C., Yamaguchi H., Ruiz A., Martinez A., Madrigal L. The E280A presenilin 1 Alzheimer mutation produces increased A beta 42 deposition and severe cerebellar pathology. Nat Med. 1996 Oct;2(10):1146–1150. doi: 10.1038/nm1096-1146. [DOI] [PubMed] [Google Scholar]
- Letourneau P. C., Cypher C. Regulation of growth cone motility. Cell Motil Cytoskeleton. 1991;20(4):267–271. doi: 10.1002/cm.970200402. [DOI] [PubMed] [Google Scholar]
- Levitan D., Doyle T. G., Brousseau D., Lee M. K., Thinakaran G., Slunt H. H., Sisodia S. S., Greenwald I. Assessment of normal and mutant human presenilin function in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14940–14944. doi: 10.1073/pnas.93.25.14940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan D., Greenwald I. Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer's disease gene. Nature. 1995 Sep 28;377(6547):351–354. doi: 10.1038/377351a0. [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E., Wasco W., Poorkaj P., Romano D. M., Oshima J., Pettingell W. H., Yu C. E., Jondro P. D., Schmidt S. D., Wang K. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995 Aug 18;269(5226):973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E., Wijsman E. M., Nemens E., Anderson L., Goddard K. A., Weber J. L., Bird T. D., Schellenberg G. D. A familial Alzheimer's disease locus on chromosome 1. Science. 1995 Aug 18;269(5226):970–973. doi: 10.1126/science.7638621. [DOI] [PubMed] [Google Scholar]
- Li X., Greenwald I. HOP-1, a Caenorhabditis elegans presenilin, appears to be functionally redundant with SEL-12 presenilin and to facilitate LIN-12 and GLP-1 signaling. Proc Natl Acad Sci U S A. 1997 Oct 28;94(22):12204–12209. doi: 10.1073/pnas.94.22.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguri G., Cecchi C., Latorraca S., Pieri A., Sorbi S., Degl'Innocenti D., Ramponi G. Alteration of acylphosphatase levels in familial Alzheimer's disease fibroblasts with presenilin gene mutations. Neurosci Lett. 1996 Jun 7;210(3):153–156. doi: 10.1016/0304-3940(96)12696-3. [DOI] [PubMed] [Google Scholar]
- Marambaud P., Ancolio K., Lopez-Perez E., Checler F. Proteasome inhibitors prevent the degradation of familial Alzheimer's disease-linked presenilin 1 and potentiate A beta 42 recovery from human cells. Mol Med. 1998 Mar;4(3):147–157. [PMC free article] [PubMed] [Google Scholar]
- Moussaoui S., Czech C., Pradier L., Blanchard V., Bonici B., Gohin M., Imperato A., Revah F. Immunohistochemical analysis of presenilin-1 expression in the mouse brain. FEBS Lett. 1996 Apr 1;383(3):219–222. doi: 10.1016/0014-5793(96)00250-5. [DOI] [PubMed] [Google Scholar]
- Murayama O., Honda T., Mercken M., Murayama M., Yasutake K., Nihonmatsu N., Nakazato Y., Michel G., Song S., Sato K. Different effects of Alzheimer-associated mutations of presenilin 1 on its processing. Neurosci Lett. 1997 Jun 20;229(1):61–64. doi: 10.1016/s0304-3940(97)00417-5. [DOI] [PubMed] [Google Scholar]
- Murphy G. M., Jr, Forno L. S., Ellis W. G., Nochlin D., Levy-Lahad E., Poorkaj P., Bird T. D., Jiang Z., Cordell B. Antibodies to presenilin proteins detect neurofibrillary tangles in Alzheimer's disease. Am J Pathol. 1996 Dec;149(6):1839–1846. [PMC free article] [PubMed] [Google Scholar]
- Page K., Hollister R., Tanzi R. E., Hyman B. T. In situ hybridization analysis of presenilin 1 mRNA in Alzheimer disease and in lesioned rat brain. Proc Natl Acad Sci U S A. 1996 Nov 26;93(24):14020–14024. doi: 10.1073/pnas.93.24.14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen W. A., Guo Q., Hartman B. K., Mattson M. P. Nerve growth factor-independent reduction in choline acetyltransferase activity in PC12 cells expressing mutant presenilin-1. J Biol Chem. 1997 Sep 5;272(36):22397–22400. doi: 10.1074/jbc.272.36.22397. [DOI] [PubMed] [Google Scholar]
- Podlisny M. B., Citron M., Amarante P., Sherrington R., Xia W., Zhang J., Diehl T., Levesque G., Fraser P., Haass C. Presenilin proteins undergo heterogeneous endoproteolysis between Thr291 and Ala299 and occur as stable N- and C-terminal fragments in normal and Alzheimer brain tissue. Neurobiol Dis. 1997;3(4):325–337. doi: 10.1006/nbdi.1997.0129. [DOI] [PubMed] [Google Scholar]
- Ratovitski T., Slunt H. H., Thinakaran G., Price D. L., Sisodia S. S., Borchelt D. R. Endoproteolytic processing and stabilization of wild-type and mutant presenilin. J Biol Chem. 1997 Sep 26;272(39):24536–24541. doi: 10.1074/jbc.272.39.24536. [DOI] [PubMed] [Google Scholar]
- Rogaev E. I., Sherrington R., Rogaeva E. A., Levesque G., Ikeda M., Liang Y., Chi H., Lin C., Holman K., Tsuda T. Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature. 1995 Aug 31;376(6543):775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T. D., Hardy J., Hutton M., Kukull W. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996 Aug;2(8):864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Seeger M., Nordstedt C., Petanceska S., Kovacs D. M., Gouras G. K., Hahne S., Fraser P., Levesque L., Czernik A. J., George-Hyslop P. S. Evidence for phosphorylation and oligomeric assembly of presenilin 1. Proc Natl Acad Sci U S A. 1997 May 13;94(10):5090–5094. doi: 10.1073/pnas.94.10.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Bronson R. T., Chen D. F., Xia W., Selkoe D. J., Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997 May 16;89(4):629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- Sherrington R., Rogaev E. I., Liang Y., Rogaeva E. A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995 Jun 29;375(6534):754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Struhl G., Greenwald I. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature. 1999 Apr 8;398(6727):522–525. doi: 10.1038/19091. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Murayama M., Takashima A., Mercken M., Nakazato Y., Noguchi K., Imahori K. Molecular cloning and expression of the rat homologue of presenilin-1. Neurosci Lett. 1996 Mar 15;206(2-3):113–116. doi: 10.1016/s0304-3940(96)12449-6. [DOI] [PubMed] [Google Scholar]
- Thinakaran G., Borchelt D. R., Lee M. K., Slunt H. H., Spitzer L., Kim G., Ratovitsky T., Davenport F., Nordstedt C., Seeger M. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996 Jul;17(1):181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Thinakaran G., Harris C. L., Ratovitski T., Davenport F., Slunt H. H., Price D. L., Borchelt D. R., Sisodia S. S. Evidence that levels of presenilins (PS1 and PS2) are coordinately regulated by competition for limiting cellular factors. J Biol Chem. 1997 Nov 7;272(45):28415–28422. doi: 10.1074/jbc.272.45.28415. [DOI] [PubMed] [Google Scholar]
- Thinakaran G., Regard J. B., Bouton C. M., Harris C. L., Price D. L., Borchelt D. R., Sisodia S. S. Stable association of presenilin derivatives and absence of presenilin interactions with APP. Neurobiol Dis. 1998 Apr;4(6):438–453. doi: 10.1006/nbdi.1998.0171. [DOI] [PubMed] [Google Scholar]
- Uchida N., Honjo Y., Johnson K. R., Wheelock M. J., Takeichi M. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J Cell Biol. 1996 Nov;135(3):767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchihara T., el Hachimi H. K., Duyckaerts C., Foncin J. F., Fraser P. E., Levesque L., St George-Hyslop P. H., Hauw J. J. Widespread immunoreactivity of presenilin in neurons of normal and Alzheimer's disease brains: double-labeling immunohistochemical study. Acta Neuropathol. 1996 Oct;92(4):325–330. doi: 10.1007/s004010050526. [DOI] [PubMed] [Google Scholar]
- Walter J., Capell A., Grünberg J., Pesold B., Schindzielorz A., Prior R., Podlisny M. B., Fraser P., Hyslop P. S., Selkoe D. J. The Alzheimer's disease-associated presenilins are differentially phosphorylated proteins located predominantly within the endoplasmic reticulum. Mol Med. 1996 Nov;2(6):673–691. [PMC free article] [PubMed] [Google Scholar]
- Wolozin B., Iwasaki K., Vito P., Ganjei J. K., Lacanà E., Sunderland T., Zhao B., Kusiak J. W., Wasco W., D'Adamio L. Participation of presenilin 2 in apoptosis: enhanced basal activity conferred by an Alzheimer mutation. Science. 1996 Dec 6;274(5293):1710–1713. doi: 10.1126/science.274.5293.1710. [DOI] [PubMed] [Google Scholar]
- Wong P. C., Zheng H., Chen H., Becher M. W., Sirinathsinghji D. J., Trumbauer M. E., Chen H. Y., Price D. L., Van der Ploeg L. H., Sisodia S. S. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature. 1997 May 15;387(6630):288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- Ye Y., Lukinova N., Fortini M. E. Neurogenic phenotypes and altered Notch processing in Drosophila Presenilin mutants. Nature. 1999 Apr 8;398(6727):525–529. doi: 10.1038/19096. [DOI] [PubMed] [Google Scholar]
- Yu G., Chen F., Levesque G., Nishimura M., Zhang D. M., Levesque L., Rogaeva E., Xu D., Liang Y., Duthie M. The presenilin 1 protein is a component of a high molecular weight intracellular complex that contains beta-catenin. J Biol Chem. 1998 Jun 26;273(26):16470–16475. doi: 10.1074/jbc.273.26.16470. [DOI] [PubMed] [Google Scholar]
- Zhou J., Liyanage U., Medina M., Ho C., Simmons A. D., Lovett M., Kosik K. S. Presenilin 1 interaction in the brain with a novel member of the Armadillo family. Neuroreport. 1997 Apr 14;8(6):1489–1494. doi: 10.1097/00001756-199704140-00033. [DOI] [PubMed] [Google Scholar]