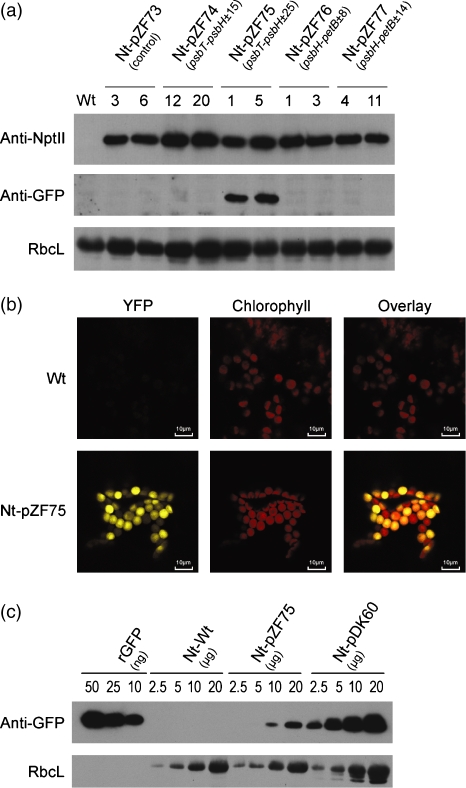
Figure 5. Foreign protein accumulation in transplastomic lines harboring various candidate processing elements between the nptII and yfp cistrons.
(a) Western blot detection of NptII and YFP protein accumulation in transplastomic lines. Total soluble proteins were separated by denaturing gel electrophoresis, blotted and probed with anti-NptII or anti-GFP antibodies. As a loading control, accumulation of the large subunit of Rubisco (RbcL) is shown. Whereas the NptII protein accumulates to comparable levels in all transplastomic lines, YFP accumulation is restricted to Nt-pZF75 lines (containing the ±25 IEE from the psbT–psbH intergenic spacer), correlating with accumulation of stable monocistronic yfp message only in these lines.
(b) Detection of YFP fluorescence in Nt-pZF75 lines by confocal laser-scanning microscopy. Yellow YFP fluorescence, red fluorescence of the chlorophyll, and the overlay of the two fluorescences are shown for the wild-type and an Nt-pZF75 transplastomic line.
(c) Comparison of YFP accumulation in Nt-pZF75 transplastomic lines with GFP accumulation in control transplastomic plants expressing GFP from the Prrn–Trps16 expression cassette (Nt-pDK60). The GFP signal in Nt-pDK60 plants is stronger than the YFP signal in Nt-pZF75 lines, which may be due to weaker recognition of YFP by the anti-GFP antibody and/or lower stability of YFP in plastids. A dilution series with purified GFP protein (rGFP) is also shown. Immunological detection of the Rubisco large subunit (RbcL) was performed as a loading control.