Figure 2.
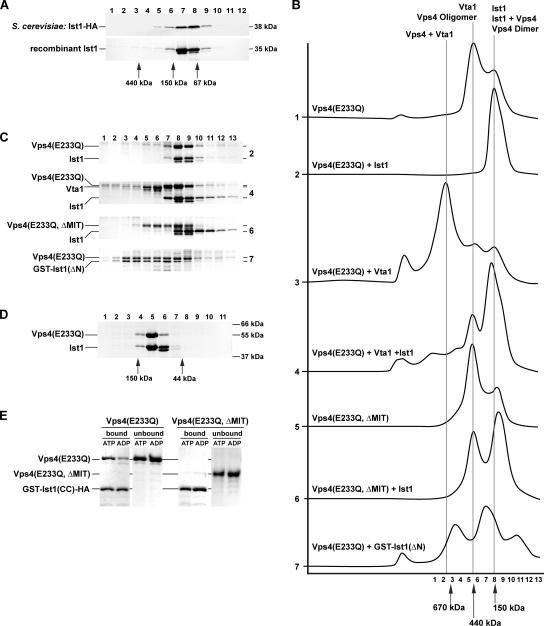
Ist1 binds to Vps4 and inhibits oligomerization of the ATPase. (A) Gel filtration analysis of cell extracts and purified recombinant protein using an S300-Sephacryl column. The resulting fractions were analyzed for the presence of Ist1-HA by Western blot and for the presence of the recombinant Ist1 by SDS-PAGE followed by Coomassie staining (top panel, extract from MBY63 pMB241; bottom panel, purified Ist1). (B) Superose S6 gel filtration analysis of purified recombinant proteins in presence of 1 mM ATP (X-axis, time; Y-axis, UV280 nm absorption). All proteins analyzed were present at 1 mg/ml in the loading sample. (C) SDS-PAGE analysis of the gel filtration fractions from the experiments in B. The sample number corresponds to the fraction number in B. The gel number indicates from which experiment in B the samples originate. (D) Gel filtration analysis using an S200-Sephacryl column of purified Ist1 and Vps4(E233Q) in presence of 1 mM ATP (1 mg/ml protein each). The fractions were analyzed by SDS-PAGE. (E) GST-Ist1(CC)-HA was immobilized on GSH-Sepharose and incubated with either Vps4(E233Q) or Vps4(E233Q, ΔMIT) in presence of 1 mM ATP or ADP. The bound and unbound fractions were analyzed by SDS-PAGE.