Figure 3.
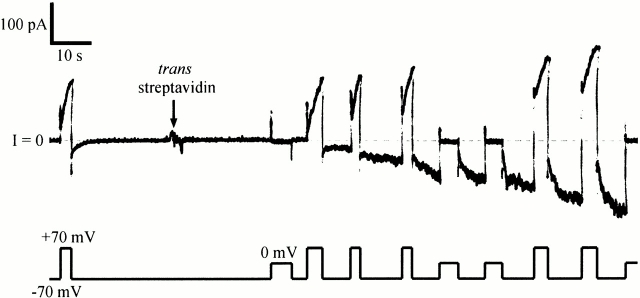
The effect of trans streptavidin on C domain with biotin attached near its amino terminus. Before the start of the record, 20 ng of biotinylated mutant 453C/CT-S without a His-tag (plus 1 μg of octyl glucoside) were added to the cis compartment. Normal gating was seen, with the conductance turning on at +70 and off at −70 mV. At the arrow, 20 μg of streptavidin was added to the trans compartment. A new conductance rapidly developed that turned on at −70 and off at 0 or +70 mV—the reverse of the normal voltage dependence. (The turn off of the “reversed” conductance during a pulse to 0 or +70 mV was evident from the decreased conductance at −70 mV just after the pulse, relative to that just before the pulse.) After streptavidin addition, there were two populations of channel in the membrane: the original population of normal-gating channels that had not yet bound trans streptavidin, and a new population of reverse-gating channels that had. This shows that the amino-terminal biotin was accessible to trans streptavidin. The solution on both sides of the membrane was 100 mM KCl, 5 mM CaCl2, 1 mM EDTA, 20 mM MES, pH 6.2. The record was filtered at 30 Hz.