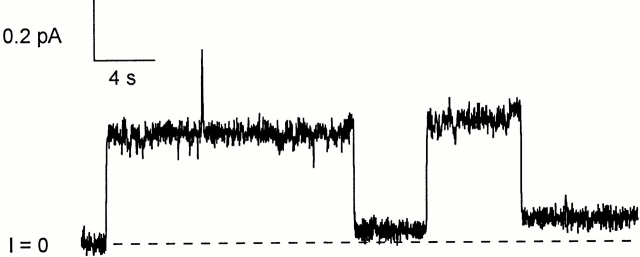
Figure 7.
The effect of trans trypsin on whole colicin Ia channels. Before the start of the record, 100 ng of whole colicin Ia were added to the cis compartment, and 5 μg of trypsin were added to the trans compartment. The record shows two channels opening at +40 mV. Each channel opened with the normal conductance (9 pS) for this salt condition, but then dropped to a low-conductance open state (0.9–1.0 pS). This decrease in conductance presumably reflects the cutting by trypsin of a site in the translocated segment and the subsequent separation of the helix 1 membrane-spanning segment from the rest of the channel. The solution on both sides of the membrane was 100 mM KCl, 5 mM CaCl2, 1 mM EDTA, 20 mM HEPES, pH 8.0. The record was filtered at 20 Hz.