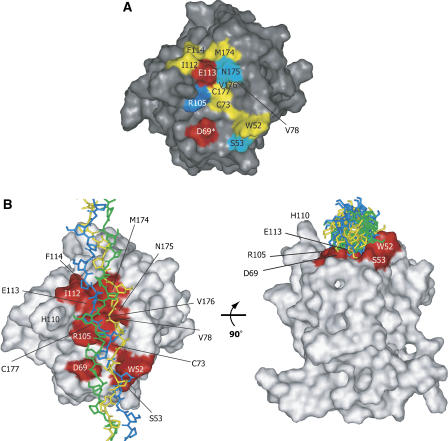
Figure 4.
The properties of the collagen-binding trench. (A) Surface representation of the binding interface of the DDR2-DS domain. The residues at the collagen-binding interface are colored according to their side-chain properties: hydrophilic residues are shown in cyan; hydrophobic residues are yellow; acidic residues are red; and the basic residue is blue. An asterisk (*) indicates that the residue was identified by the mutation, but did not display TCS signal intensity reduction. (B) The proposed model of the DDR2-DS domain–triple-helical peptide, obtained by using the PatchDock software (Schneidman-Duhovny et al, 2003) (http://bioinfo3d.cs.tau.ac.il/PatchDock). The model peptide of collagen with the sequence (GPO)12 (PDB code 2CLG; Chen et al, 1991) was used as the ligand, with each of the 20 conformers of the DDR2-DS domain as the receptor. The solution with the highest score among the top 20 solutions that satisfied the NMR and mutagenesis data was chosen. The collagen-binding residues of the DDR2-DS domain are colored red. The three model peptides of collagen are shown as stick models colored green, blue, and yellow, respectively.