Abstract
Both endogenous and exogenous opioids modulate blood pressure and cardiac function by stimulating cardiac synthesis of atrial natriuretic factor (ANF) and brain natriuretic peptide (BNP). Since morphine crosses the placental barrier, it could alter the ANF-BNP system in the fetal heart. The aim of this study was to characterize cardiac natriuretic peptides in normal rat development and in rats prenatally exposed to morphine.
Female rats received either saline or morphine (10 or 20 mg kg−1 day−1) via osmotic minipumps during gestation. The effects of this treatment were investigated in offspring at 1, 4 and 22 days of age.
During maturation, atrial ANF and ANF mRNA increased by 3-fold from birth to 3 weeks of age, but BNP and BNP mRNA tended to decrease. In the ventricles, both ANF and BNP content decreased at 3 weeks after birth, from 25.11 ± 3.6 to 0.81 ± 0.1 ng (P < 0.001), and from 3.36 ± 0.33 to 0.19 ± 0.01 ng (P < 0.001), respectively. However, whereas ventricular ANF mRNA decreased, BNP mRNA levels did not change during maturation. Prenatal exposure to morphine significantly increased ANF content in the left atria of 22-day-old rats, and in the right atria of 1-, 4- and 22-day-old rats compared with age-matched saline controls. In contrast, prenatal exposure to 20 mg kg−1 day−1 morphine significantly inhibited BNP and BNP mRNA in the ventricles at all ages studied.
These observations suggest that alterations in mRNA synthesis or stability and/or post-translational processing of ANF and BNP occur in the heart during maturation, and that prenatal exposure to morphine alters cardiac production, and possibly release, of both peptides.
Atrial natriuretic factor (ANF) and brain natriuretic peptide (BNP) are two peptides, encoded by different genes, that are synthesized by the atria and ventricles of the heart, secreted and then released into the circulation. In adults, ANF and BNP have significant effects on cardiovascular, fluid and electrolyte homeostasis (De Bold & Flynn, 1983; Cheung, Dickerson, Ashby, Brown & Brown, 1994). In newborn animals, fluid homeostasis shifts to a new equilibrium that is characterized by a reduction of total blood and extracellular fluid volumes. Substantial modifications in ANF gene expression have been shown to occur during the dramatic circulatory changes that take place during the perinatal period. Atrial ANF increases in the 2 week period after birth, but ventricular ANF decreases to levels near those found in adults (Wu, Deschepper & Gardner, 1988; Dolan, Young, Khoury & Dobrozski, 1989). BNP kinetics during this period are not clear. However, previous studies have shown that during development, cardiac expression of BNP and ANF genes are discordantly regulated (Dagnino, Drouin & Nemer, 1991; Takahashi, Allen & Izumo, 1992).
Opioid drugs are widely used as analgesics in patients suffering from pain, including that of labour (Skibsted & Lange, 1992). Morphine therapy during human pregnancy is recommended for the reversal of severe maternal pulmonary hypertension (Leduc, Kirshon, Diaz & Cotton, 1990). Also, when administered during pregnancy, morphine brings morphological (a decrease of total body weight and modification of brain weight) and behavioural changes in the fetus (Kirby & Holtzman, 1982). Diffusion from maternal to fetal compartments is facilitated because morphine is a lipophilic drug, whereas the placental membrane is lipoproteic. Studies have shown that in the rat, several opioids cross the placenta towards the end of gestation (around the nineteenth and twenty-first days). Kirby (1979) showed that morphine crosses the placenta with less difficulty as gestation progresses, a finding consistent with the morphological development of the placenta: the placental barrier is relatively thick in early pregnancy, but it becomes thinner as pregnancy progresses.
Morphine is a potent stimulus of ANF release (Gutkowska et al. 1986; Tang, Xie, Cie, Gao & Chang, 1987; Vollmar, Arendt & Schulz, 1987). At low intravenous or intracerebroventricular doses, opioids increase plasma ANF and the effects are reversed by naloxone, an opioid antagonist (Gutkowska et al. 1986). In addition, basal levels of ANF are partially inhibited by naloxone, which implicates endogenous opioids in ANF regulation (Gutkowska et al. 1986). Morphine also stimulates BNP release, but to a lesser degree (Aburaya, Suzuki, Minamino, Kanagwa, Tanaka & Matsuo, 1991; Thibault, Charbonneau, Bilodeau, Schiffrin & Garcia, 1992). The mechanisms by which morphine increases ANF and BNP have not yet been elucidated, but it appears that the regulation of ANF is mediated, at least in part, by opioid receptors, without a concomitant rise in blood pressure (Gutkowska, Strick, Pan & McCann, 1993).
Therefore, based on the above reports, and on studies showing that acute morphine treatment enhances ANF synthesis in the atria of adult rats (Fukui, Iwao, Nakamura, Tamaki & Abe, 1991) we hypothesized that maternal exposure to morphine may directly alter the cardiac natriuretic peptides, ANF and BNP, during ontogeny. Consequently, studies were performed to characterize the maturation-induced changes in cardiac natriuretic peptides in rat offspring from birth to 3 weeks of age, and to assess the influences of prenatal treatment of the mother with morphine upon the changes of cardiac ANF and BNP at the protein and mRNA levels.
METHODS
Female Sprague-Dawley rats (weight, 175-200 g; Charles River, St Constant, Quebec) were mated. The presence of spermatozoids in vaginal smears was considered as indicative of the first day of pregnancy. On the fifth day of gestation, the rats received either saline vehicle (0.9 % NaCl), or morphine at 10, 20 or 40 mg kg−1 day−1 (prepared from sulphate powder in 0.9 % NaCl). The doses of morphine were selected following the method of Fujinaga & Mazze (1988). The solutions were administered via Alzet osmotic 2ML2 minipumps (Alza Corporation, Palo Alto, CA, USA), which delivered at a rate of 5 ml h−1 over 14 days. The solutions were introduced into the pumps with a syringe and a blunt-tipped filling tube. The pump was then placed in saline at 37°C for 4 h to prevent clot formation. Finally, the pumps were implanted subcutaneously in rats anaesthetized by inhalation of a mixture of oxygen and 5 % enflurane. The rats were then placed individually in cages, with food and water ad libitum. Temperature (18-19°C) and humidity (65 %) were controlled, and the animals were kept under a 12 h light-dark cycle.
After birth, the offspring were counted and weighed. The offspring were killed by decapitation at 1, 4 and 22 days of age. The experiments were performed in accordance with the guidelines of the Canadian Council on Animal Care.
Determination of ANF and BNP
Left and right atria and ventricles were excised, immediately frozen in liquid nitrogen and kept at -70°C until use. The tissues were homogenized in cold acetic acid (0.1 mol l−1) containing protease inhibitors (final concentrations): pepstatin A, 0.5 × 10−5 mol l−1; phenylmethylsulphonic fluoride (PMSF), 10−5 mol l−1; and EDTA, 10−5 mol l−1, then centrifuged at 30 000 g for 20 min at 4°C. The supernatant was aliquoted and stored at -70°C. Protein concentration was determined in tissue homogenates using bovine serum albumin (BSA) standards.
ANF and BNP were quantified by specific radioimmunoassays (RIA) developed in our laboratory (Gutkowska, 1987; Guillaume, Jankowski, Gutkowska & Gianoulakis, 1996). ANF and BNP were radiolabelled with 125I-Na and lactoperoxidase, then purified by HPLC. Antibodies generated against the synthetic ANF peptide (Arg101-Tyr126) recognize circulating ANF (Ser99-Tyr126) as well as the 126 amino acid prohormone with 100 % cross-reactivity (Gutkowska, 1987), but do not recognize BNP. Rat BNP antibody and standards were purchased from Peninsula Laboratories (Belmont, CA, USA). The BNP antiserum does not recognize ANF or ANF prohormone. The sensitivity of the ANF assay was 3 pg per tube and that of BNP assay 6 pg per tube. The intra- and inter-assay coefficients of variation for ANF were < 10 % and 15 %, respectively, and for BNP were < 13 % and 15 %, respectively.
Northern blot
Northern blot analysis was performed as previously detailed (Guillaume et al. 1996). In brief, ventricular (10 μg) or atrial (2 μg) RNA were separated by electrophoresis through 1.5 % agarose gels containing 0.22 M formaldehyde. Immobilized RNA samples were hybridized with random-primed α-32P-labelled cDNA probes corresponding to ANF and α-tubulin mRNA sequences. (The Pst I- digested 660 bp fragment from the plasmid ANF clone (kindly provided by Dr Mona Nemer, IRCM, Montreal, Canada) was used as the ANF probe.) Probes consisting of a 347 bp fragment of BNP cDNA and a 550 bp fragment of α-tubulin cDNA were generated by reverse transcription of rat atrial mRNA and amplification of the resulting cDNA by the polymerase chain reaction as described previously (Dagnino et al. 1991). After hybridization, washed membranes were exposed on phosphor-sensitive cassettes, then scanned on the PhosphorImager (Molecular Dynamics, Sunnyvale, CA, USA). Radioactive bands were measured with Image-Quant software (Molecular Dynamics). Percentage (or fold) increases in ANF mRNA or BNP mRNA were normalized to α-tubulin and compared with saline-receiving controls. In several experiments, nylon membranes were hybridized sequentially with α-tubulin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) probes. However, similar results were obtained when blots were normalized to either control probe.
Statistical analysis
Significance among the various groups was evaluated by two-way analysis of variance (ANOVA), with age as the first factor and dose as the second factor, followed by the B value of Tukey's multiple comparison test. A difference of less than 0.05 was considered significant. Values are expressed as means ±s.e.m.
RESULTS
Pregnancy rate, mean gestational age and the number of live births per litter were not different in rats treated with saline, or morphine at doses of 10, 20 and 40 mg kg−1 day−1. The offspring of mothers treated with morphine at doses of 10 and 20 mg kg−1 day−1 tended to be of lower weight than age-matched controls, but the observed differences were not statistically significant. However, the mean birth weight in the offspring of rats treated with a morphine dose of 40 mg kg−1 day−1 was significantly (P < 0.001) lower than that in the control group (1 day, 5.45 ± 0.09 vs. 6.45 ± 0.1 g; 4 days, 5.76 ± 0.15 vs. 11.12 ± 0.21 g; and 22 days, 45.19 ± 0.67 vs. 64.96 ± 1.07 g). Since the birth weights were altered by the 40 mg dose, this dose was dropped and further studies were performed using only 10 and 20 mg doses.
ANF and BNP concentration in rat atria
Tissue concentrations of ANF were measured in the atria and ventricles of rats at 1, 4 and 22 days of age, by a specific radioimmunoassay (RIA). Table 1 shows that the ANF content of right and left atria were not different. In both atria, ANF content increased by 3- to 4-fold from 1 to 22 days, in saline- as well as in morphine-treated rats (P < 0.001). However, compared with corresponding age-matched saline-treated controls, prenatal exposure to morphine increased ANF in both left and right atria at all ages. For left atria, this difference was significant (6942 ± 576 vs. 9855 ± 1052 ng (mg protein)−1; n= 16; P < 0.05) at 22 days of age. In right atria the difference was significant at 1 day (1935 ± 214 vs. 3208 ± 271 ng (mg protein)−1; n= 16; P < 0.05): 4 days (2797 ± 289 vs. 3846 ± 485 ng (mg protein)−1; n= 16; P < 0.05) and 22 days (6308 ± 970 vs. 8795 ± 802 ng (mg protein)−1; n= 16; P < 0.05) of age.
Table 1.
Atrial natriuretic factor (ANF) and brain natriuretic peptide (BNP) in cardiac atria and ventricles of 1-, 4- and 22-day-old rats prenatally exposed to morphine treatment
| ANF ng (mg protein)−1 | BNP ng (mg protein)−1 | ||||
|---|---|---|---|---|---|
| Day | Saline | Morphine | Saline | Morphine | |
| Right atria | 1 | 1935 ± 214 | 3208 ± 271 ‡ | 54.9 ± 9.1 | 58.5 ± 2.6 |
| 4 | 2797 ± 289 * | 3846 ± 485 *† | 46.1 ± 7.1 | 34.4 ± 2.0 ** | |
| 22 | 6308 ± 970 ** | 8795 ± 802 **† | 38.3 ± 7.8 | 31.4 ± 3.1 ** | |
| Left atria | 1 | 1954 ± 311 | 2576 ± 175 | 58.6 ± 10.7 | 84.4 ± 5.2 † |
| 4 | 2853 ± 282 * | 3845 ± 478 * | 46.5 ± 9.7 | 34.1 ± 3.6 ** | |
| 22 | 6942 ± 576 ** | 9855 ± 1052 **† | 42.7 ± 7.5 | 26.5 ± 4.3 ** | |
| Ventricles | 1 | 25.1 ± 3.6 | 33.6 ± 3.4 | 3.36 ± 0.32 | 2.11 ± 0.14 † |
| 4 | 55.4 ± 8.2 ** | 66.2 ± 8.9 ** | 2.74 ± 0.50 ** | 1.39 ± 0.31 **‡ | |
| 22 | 0.8 ± 0.1 ** | 1.0 ± 0.2 ** | 0.19 ± 0.03 ** | 0.12 ± 0.02 †** | |
Values represent means ±s.e.m., n= 16 rats per group.
P < 0.05
P < 0.001, 4- or 22-day-old vs. 1-day-old rats.
P < 0.05
P < 0.001, morphine-treated vs. corresponding saline-treated controls.
Table 1 also shows that whereas BNP content was not altered by age in saline-treated rats, treatment with morphine resulted in a significant decrease in BNP content in the left atria, from 84.4 ± 5.2 ng (mg protein)−1 at 1 day to 34.1 ± 3.6 and 26.5 ± 4.3 ng (mg protein)−1 at 4 and 22 days, respectively (n= 8, P < 0.001); and in the right atria from 58.5 ± 2.6 ng (mg protein)−1 at 1 day to 34.4 ± 2.0 and 31.4 ± 3.1 ng (mg protein)−1 at 4 and 22 days, respectively (n= 8, P < 0.001). Moreover, morphine treatment increased left atrial BNP content in 1-day-old rats compared with corresponding controls (n= 8, P < 0.05).
ANF and BNP in rat ventricles
A peculiar pattern of changes in ANF content was seen in ventricles during maturation (Table 1). After a significant increase from 1 to 4 days (25.1 ± 3.6 vs. 55.4 ± 8.2 ng (mg protein)−1; P < 0.01), the ANF content dropped sharply between 4 and 22 days to 0.8 ± 0.1 ng (mg protein)−1 (P < 0.01). Prenatal exposure to morphine did not alter the developmental changes in ventricular ANF between 1 and 4 days, but at 22 days ventricular ANF content was higher than in corresponding saline-treated rats (1.0 ± 0.2 vs. 0.8 ± 0.1 ng (mg protein)−1, P < 0.05).
BNP content in ventricles decreased with age in both saline- and morphine-treated rats (Fig. 1). BNP in saline-treated rats decreased from 3.36 ± 0.32 ng (mg protein)−1 at 1 day to 2.74 ± 0.5 ng (mg protein)−1 at 4 days, and to 0.19 ± 0.03 ng (mg protein)−1 at 22 days (P < 0.05 in all age groups). Morphine exposure resulted in a similar age-dependent decrease in ventricular BNP (day 1, 2.11 ± 0.14; day 4, 1.39 ± 0.31; day 22, 0.12 ± 0.02 ng (mg protein)−1. However, BNP in ventricles of morphine-treated rats, was significantly lower than that in age-matched saline controls. The effect of morphine on BNP content was dose dependent since a lower dose of 10 mg kg−1 day−1 also lowered ventricular BNP content (day 1, 2.59 ± 0.35; day 4, 1.74 ± 0.14; day 22, 0.18 ± 0.01 ng (mg protein)−1), which was significant at 1 and 4 days (P < 0.05).
Figure 1. Effect of prenatal treatment with morphineon ventricular levels of atrial natriuretic factor (ANF) and brain natriuretic peptide (BNP).
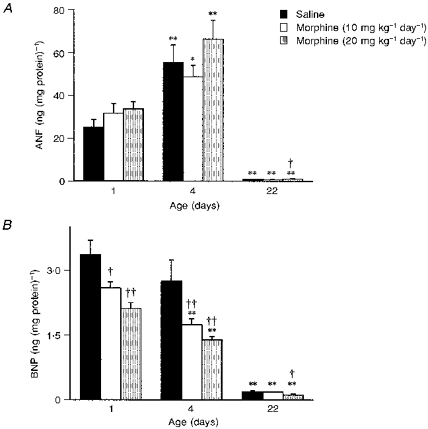
Rats were treated prenatally with morphine (20 mg kg−1 day−1 and 10 mg kg−1day−1) or saline. Subsequent experiments were performed after birth in 1-, 4- and 22-day-old rats (n= 16 in each group). *P < 0.05, **P < 0.001, 4- or 22-day-old vs. 1-day-old rats; †P < 0.05, ††P < 0.001, morphine-treated vs. corresponding saline-treated controls.
ANF and BNP gene expression in atria and ventricles
The ANF and BNP mRNA levels were evaluated by Northern blot analysis. The effect of morphine treatment (20 mg kg−1 day−1) during gestation was evaluated vs. saline-matched controls. To quantify the changes in mRNA levels, the Northern blot signals were scanned and normalized to the hybridization signal of α-tubulin mRNA. Figure 2 shows that ANF mRNA levels of saline-treated control animals increased in the left atria from 1 to 22 days of age, in parallel with the observed increase in tissue ANF content. In contrast, left atrial BNP mRNA decreased between 1 and 22 days of age. Prenatal exposure to morphine stimulated left atrial ANF mRNA in 1-day-old rats and decreased BNP mRNA in 4-day-old rats compared with age-matched controls (n= 4 for each group; P < 0.05).
Figure 2. Northern blot of ANF mRNA and BNP mRNA from left atria of rats prenatally treated with morphine (M; 20 mg kg−1 day−1) or saline (S) vehicle.
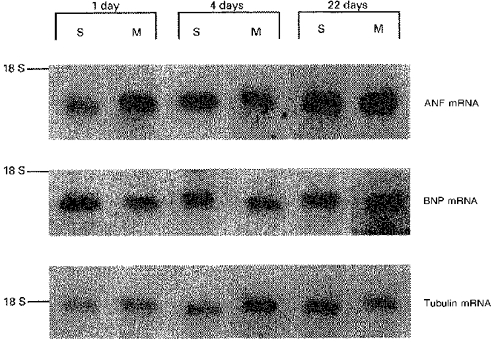
Experiments were performed on 1-, 4- and 22-day-old rats (n= 4 in each group). Representative bands of the mRNA hybridized to 32P-labelled cDNA probes are shown vs. electrophoretic position of ribosomal 18 S RNA. The PhosphorImager scan of 0.9 kb ANF or 0.9 kb BNP bands was normalized to the signal obtained with 1.6 kb tubulin bands.
In right atria, ANF mRNA levels increased with age, but not with morphine treatment during gestation. Age and morphine treatment had no effect on the levels of BNP mRNA in these rats (data not shown).
In the ventricles, a reduction in ANF was seen at the level of gene expression, with ANF mRNA decreasing between 1 and 22 days of age. However, BNP mRNA decreased only at 4 days of age. On the other hand, whereas prenatal exposure to morphine did not affect the developmental changes in ventricular ANF mRNA, it resulted in a significant dose-dependent decrease in BNP mRNA (Fig. 3).
Figure 3. Northern blot analysis of ventricular ANF and BNP mRNA.
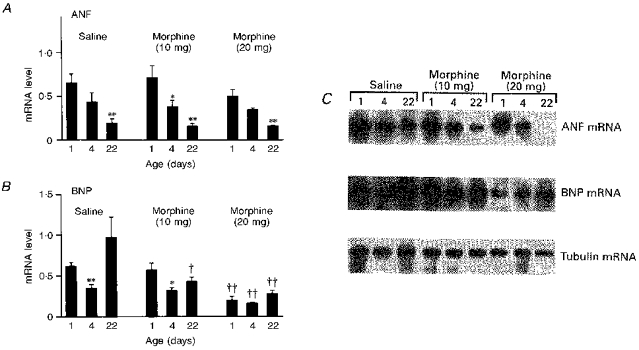
A and B show the effect of prenatal treatment of rats with morphine (20 mg kg−1 day−1 and 10 mg kg−1 day−1) or saline vehicle on ANF and BNP mRNA, respectively. Representative bands of hybridized mRNA are presented in C. Experiments were performed after birth in 1-, 4- and 22-day-old rats (n= 8 in each group). Values represent means ±s.e.m.; *P < 0.05, **P < 0.001, 4- or 22-day-old vs. 1-day-old rats; †P < 0.05, ††P < 0.001, morphine treatment vs. corresponding saline-treated controls.
DISCUSSION
In this study, we tested the hypothesis that chronic administration of morphine to rats during gestation would stimulate cardiac natriuretic peptide production and gene expression in the offspring. Our results show that normal postnatal development was associated with increased atrial ANF and ANF mRNA, but that ventricular ANF decreased by 3 weeks after birth; this decrease was confirmed at the level of expression. On the other hand, BNP decreased with postnatal development in both atria and ventricles. Furthermore, prenatal exposure to morphine resulted in an increase in atrial ANF, but a decrease in ventricular BNP and BNP mRNA.
ANF is present as a circulating hormone during fetal life, when fetal plasma ANF levels are consistently higher than maternal levels (Wei et al. 1987; Ervin et al. 1988). The observed dichotomy between fetal and maternal ANF levels suggests that ANF is probably secreted independently by the fetus and the mother. Elevated plasma ANF levels in human newborn infants between days 3 and 5 after birth are associated with diuresis, natriuresis and weight loss (Gemelli, Mami, De Luca, Stelitano, Bonaccorsi & Martino, 1991). The mechanism of elevated plasma ANF is not known, but since atrial ANF synthesis is low at birth (Wei et al. 1987), it seems likely that the ventricular contribution to plasma ANF may be increased in neonates. In the present study, ventricular ANF was indeed enhanced in 4-day-old rats, consistent with the observation of Wu et al. (1988). However, we also observed a transient decrease in ventricular BNP mRNA on day 4, an effect that disappeared by day 22.
In adult rats, BNP is co-secreted, in part, with ANF from atrial granules after stimulation with morphine (Thibault et al. 1992). Two types of granules have been reported: type I which contains ANF alone, and type II, which contains both ANF and BNP (Hasegawa et al. 1991). In the present study, the type of atrial granule has not been determined. However, since BNP content and BNP mRNA did not increase in atria during maturation, it seems that the increased number of atrial granules is limited to type I.
The present study shows that maturation modifies ANF and BNP in opposite manners. In the right and left atria, ANF increases but BNP decreases. These changes parallel the changes in ANF and BNP gene expression. In the ventricles, both ANF and BNP show marked reductions at 3 weeks after birth. These reductions may be explained in part by the disappearance of secretory granules of ANF and BNP, and by the small ventricular storage capacity of these peptides during development (Bloch, Seidman, Naftilan, Fallon & Seidman, 1986). Ventricular ANF mRNA decreased without changes in BNP mRNA, implying that ANF and BNP genes are discordantly regulated during development. The altered regulation may be influenced by differences in the sequences of the ANF and BNP genes, the rate of gene transcription and differential stabilization of the mRNA (Roy & Flynn, 1990).
In the present study, chronic prenatal treatment with morphine was associated with altered synthesis of the natriuretic peptides. Morphine resulted in lower BNP mRNA levels in the ventricles, but was less effective in the atria. In contrast, morphine did not alter ANF mRNA in ventricles, but increased it in atria. These findings are consistent with acute studies in which morphine resulted in a 50-fold increase in plasma ANF within 2-120 min, and a 4-fold increase in plasma BNP within 15-45 min (Thibault et al. 1992). The mechanisms involved in increasing ANF include massive secretion from atrial stores. The release of BNP did not parallel that of ANF, indicating that BNP originated not only from the atrial granules, but also from other tissues such as the ventricles (Thibault et al. 1992). There is a consensus that the most important stimulus for ANF release is activation of stretch receptors within the cardiac atria. However, we previously reported that stimulation of central opioid receptors induces ANF release in the absence of haemodynamic changes (Gutkowska et al. 1993). On the other hand, there is evidence that dynorphin can stimulate ANF release by a direct action on the atria (Stash, Grote, Kazda & Hirth, 1989). These observations suggest that the regulation of ANF release by morphine is more complex.
Prenatal exposure to morphine may alter cardiac ANF and BNP synthesis via transplacental passage and activation of opioid receptors in the fetal heart. The presence of opioid receptors in the rat cardiac sarcolemma (Ventura, Spurgeon, Lakatta, Guarnieri & Capogrossi, 1992), myocardial naloxone uptake and naloxone-enhanced contractile function (Collins & DiCarlo, 1993) clearly indicate a direct cardiac action of opioid peptides. An interesting finding is that the μ-subtype of opioid receptors that transduce morphine signals are expressed only at early periods of heart ontogeny in rats, and are absent in animals over 2 weeks of age (Zimlichman et al. 1996). This observation suggests that μ-receptors, selectively specific for morphine, may be present in the fetal heart.
ANF and BNP are present in the heart in early fetal life, and in humans they are detected as early as the twelfth week of pregnancy (Hyett, Brizot, Vonkaisenberg, McKie, Farzaneh & Nicolaides, 1996). In the mouse, at 9.5 days of gestation very high levels of ANF and BNP mRNA are observed in fetal heart, but the predominant site of expression is the ventricles, rather than the atria (Cameron, Aitken, Ellmers, Kennedy & Espiner, 1996). The high levels of ANF and BNP expression observed in fetal heart suggest a function for these peptides in fetal water and salt homeostasis. Moreover Cameron et al. (1996) observed that fluctuations in ANF and BNP mRNA levels are associated with important stages of development of the embryonic heart, suggesting their involvement in this process. Therefore, treatment with morphine during gestation, causing atrial ANF and in particular ventricular BNP levels to alter, might have patho-physiological implications both for the embryo and, possibly, postnatally.
In summary, we conclude that during development differences exist between ANF and BNP in synthesis, storage, and processing patterns in rat ventricles and atria. ANF synthesis in the atria of offspring may be stimulated by chronic prenatal treatment with morphine, but ventricular BNP content decreases and this decrease is observed at the level of BNP mRNA. Further studies are required to elucidate the functional significance of these changes.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Céline Coderre and Nathalie Charron. This work was supported by grants from The Medical Research Council of Canada (grant nos MT-10337 and MT-11674) and the Heart and Stroke Foundation of Canada to J. G.
References
- Aburaya M, Suzuki E, Minamino N, Kangawa K, Tanaka K, Matsuo H. Concentration and molecular forms of brain natriuretic peptide in rat plasma and spinal cord. Biochemical and Biophysical Research Communications. 1991;177:40–47. doi: 10.1016/0006-291x(91)91945-9. [DOI] [PubMed] [Google Scholar]
- Bloch KD, Seidman JG, Naftilan JD, Fallon JT, Seidman CE. Neonatal atria and ventricles secrete atrial natriuretic factor via tissue-specific secretory pathways. Cell. 1986;47:695–702. doi: 10.1016/0092-8674(86)90512-x. [DOI] [PubMed] [Google Scholar]
- Cameron VA, Aitken GD, Ellmers LJ, Kennedy MA, Espiner EA. The sites of gene expression of atrial, brain, and C-type natriuretic peptides in mouse fetal development: Temporal changes in embryos and placenta. Endocrinology. 1996;137:817–823. doi: 10.1210/endo.137.3.8603590. [DOI] [PubMed] [Google Scholar]
- Cheung BMY, Dickerson JEC, Ashby MJ, Brown MJ, Brown J. Effects of physiological increments in human α-atrial natriuretic peptide and human brain natriuretic peptide in normal male subjects. Clinical Science. 1994;86:723–730. doi: 10.1042/cs0860723. [DOI] [PubMed] [Google Scholar]
- Collins H, DiCarlo S. Attenuation of postexertional hypotension by cardiac afferent blockade. American Journal of Physiology. 1993;265:H1179–1183. doi: 10.1152/ajpheart.1993.265.4.H1179. [DOI] [PubMed] [Google Scholar]
- Dagnino L, Drouin J, Nemer M. Differential expression of natriuretic peptide genes in cardiac and extracardiac tissues. Molecular Endocrinology. 1991;5:1292–1300. doi: 10.1210/mend-5-9-1292. [DOI] [PubMed] [Google Scholar]
- De Bold A, Flynn TG. Cardionatrin I - a novel heart peptide with potent diuretic and natriuretic properties. Life Science. 1983;33:297–302. doi: 10.1016/0024-3205(83)90390-9. 10.1016/0024-3205(83)90390-9. [DOI] [PubMed] [Google Scholar]
- Dolan L, Young C, Khoury JC, Dobrozski DJ. Atrial natriuretic factor during the perinatal period: equal depletion in both atria. Pediatric Research. 1989;25:339–341. doi: 10.1203/00006450-198904000-00005. [DOI] [PubMed] [Google Scholar]
- Ervin M, Ross M, Castro R, Sherman D, Lam R, Castro L, Leake R, Fisher DA. Ovine fetal and adult atrial natriuretic factor metabolism. American Journal of Physiology. 1988;254:R40–46. doi: 10.1152/ajpregu.1988.254.1.R40. [DOI] [PubMed] [Google Scholar]
- Fujinaga M, Mazze R. Teratogenic and postnatal developmental studies of morphine in Sprague-Dawley rats. Teratology. 1988;38:401–410. doi: 10.1002/tera.1420380502. [DOI] [PubMed] [Google Scholar]
- Fukui K, Iwao H, Nakamura H, Tamaki T, Abe Y. Effects of water deprivation and morphine administration on atrial natriuretic peptide mRNA levels in rat auricles. Japanese Journal of Pharmacology. 1991;57:45–50. doi: 10.1254/jjp.57.45. [DOI] [PubMed] [Google Scholar]
- Gemelli M, Mami C, De Luca F, Stelitano L, Bonaccorsi P, Martino F. Atrial natriuretic peptide and renin-aldosterone relationship in healthy newborn infants. Acta Paediatrica Scandinavica. 1991;80:1123–1133. doi: 10.1111/j.1651-2227.1991.tb11799.x. [DOI] [PubMed] [Google Scholar]
- Guillaume P, Jankowski M, Gutkowska J, Gianoulakis C. Effect of chronic ethanol consumption on the brain natriuretic system of Wistar-Kyoto (WKY) and Spontaneously Hypertensive (SHR) rats. European Journal of Pharmacology. 1996;316:49–58. doi: 10.1016/s0014-2999(96)00644-9. 10.1016/S0014-2999(96)00644-9. [DOI] [PubMed] [Google Scholar]
- Gutkowska J. Radioimmunoassay for atrial natriuretic factor. Nuclear Medicine and Biology. 1987;14:323–331. doi: 10.1016/0883-2897(87)90009-2. [DOI] [PubMed] [Google Scholar]
- Gutkowska J, Racz K, Garcia R, Thibault G, Kuchel O, Genest J, Cantin M. The morphine effect on plasma ANF. European Journal of Pharmacology. 1986;131:91–94. doi: 10.1016/0014-2999(86)90519-4. 10.1016/0014-2999(86)90519-4. [DOI] [PubMed] [Google Scholar]
- Gutkowska J, Strick DM, Pan L, McCann SM. Effect of morphine on urine output: possible role of atrial natriuretic factor. European Journal of Pharmacology. 1993;242:7–13. doi: 10.1016/0014-2999(93)90003-z. 10.1016/0014-2999(93)90003-Z. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Fujiwara H, Itoh H, Nakao K, Fujiwara T, Imura H, Kawai C. Light and electron microscopic localization of brain natriuretic peptide in relation to atrial natriuretic peptide in porcine atrium. Circulation. 1991;84:1203–1209. doi: 10.1161/01.cir.84.3.1203. [DOI] [PubMed] [Google Scholar]
- Hyett J, Brizot M, Vonkaisenberg C, McKie A, Farzaneh F, Nicolaides KH. Cardiac gene expression of atrial natriuretic peptide and brain natriuretic peptide in trisomic fetuses. Obstetrics and Gynecology. 1996;87:506–510. doi: 10.1016/0029-7844(95)00486-6. 10.1016/0029-7844(95)00486-6. [DOI] [PubMed] [Google Scholar]
- Kirby M. Morphine in fetuses after maternal injection: increasing concentration with advancing gestational age. Proceedings of the Society of Experimental Biology and Medicine. 1979;162:287–290. doi: 10.3181/00379727-162-40666. [DOI] [PubMed] [Google Scholar]
- Kirby M, Holtzman S. Effects of chronic opiate administration on spontaneous activity of fetal rats. Pharmacology, Biochemistry and Behaviour. 1982;16:263–269. doi: 10.1016/0091-3057(82)90159-9. 10.1016/0091-3057(82)90159-9. [DOI] [PubMed] [Google Scholar]
- Leduc L, Kirshon B, Diaz SF, Cotton D. Intrathecal morphine analgesia and low-dose dopamine for oliguria in severe maternal pulmonary hypertension. Journal of Reproductive Medicine. 1990;35:727–729. [PubMed] [Google Scholar]
- Roy R, Flynn TG. Organization of the gene for iso-rANP, a rat B-type natriuretic peptide. Biochemical and Biophysical Research Communications. 1990;171:416–423. doi: 10.1016/0006-291x(90)91409-l. [DOI] [PubMed] [Google Scholar]
- Skibsted L, Lange AP. The need for pain relief in uncomplicated deliveries in an alternative birth center compared to an obstetric delivery ward. Pain. 1992;48:183–186. doi: 10.1016/0304-3959(92)90057-I. 10.1016/0304-3959(92)90057-I. [DOI] [PubMed] [Google Scholar]
- Stash JP, Grote H, Kazda S, Hirth C. Dynorphin stimulates the release of ANP from isolated atria. European Journal of Pharmacology. 1989;159:101–102. doi: 10.1016/0014-2999(89)90050-2. 10.1016/0014-2999(89)90050-2. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Allen PD, Izumo S. Expression of A-, B-, and C-type natriuretic peptide genes in failing and developing human ventricles - Correlation with expression of the Ca2+-ATPase gene. Circulation Research. 1992;71:9–17. doi: 10.1161/01.res.71.1.9. [DOI] [PubMed] [Google Scholar]
- Tang J, Xie CW, Cie XZ, Gao XM, Chang JK. Dynorphin A (1–10) amide stimulates release of atrial natriuretic peptide (ANP) from rat atrium. European Journal of Pharmacology. 1987;143:315–321. doi: 10.1016/0014-2999(87)90325-6. 10.1016/0014-2999(87)90455-9. [DOI] [PubMed] [Google Scholar]
- Thibault G, Charbonneau C, Bilodeau J, Schiffrin EL, Garcia R. Rat brain natriuretic peptide is localized in atrial granules and released into the circulation. American Journal of Physiology. 1992;263:R301–309. doi: 10.1152/ajpregu.1992.263.2.R301. [DOI] [PubMed] [Google Scholar]
- Ventura C, Spurgeon H, Lakatta EG, Guarnieri C, Capogrossi MC. κ- and δ-opioid receptor stimulation affects cardiac myocyte function and Ca2+ release from an intracellular pool in myocytes and neurons. Circulation Research. 1992;70:66–81. doi: 10.1161/01.res.70.1.66. [DOI] [PubMed] [Google Scholar]
- Vollmar A, Arendt R, Schultz R. The effect of opioids on rat plasma atrial natriuretic peptide. European Journal of Pharmacology. 1987;143:315–321. doi: 10.1016/0014-2999(87)90455-9. 10.1016/0014-2999(87)90455-9. [DOI] [PubMed] [Google Scholar]
- Wei Y, Rodi C, Day M, Wiegand R, Needleman L, Cole B, Needleman P. Developmental changes in the rat atriopeptin hormonal system. Journal of Clinical Investigation. 1987;79:1325–1329. doi: 10.1172/JCI112957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Deschepper C, Gardner D. Perinatal expression of the atrial natriuretic factor gene in rat cardiac tissue. American Journal of Physiology. 1988;255:E388–396. doi: 10.1152/ajpendo.1988.255.3.E388. [DOI] [PubMed] [Google Scholar]
- Zimlichman R, Gefel D, Eliahou H, Matas Z, Rosen B, Gass S, Ela C, Eilam Y, Vogel Z, Barg J. Expression of opioid receptors during heart ontogeny in normotensive and hypertensive rats. Circulation. 1996;93:1020–1025. doi: 10.1161/01.cir.93.5.1020. [DOI] [PubMed] [Google Scholar]


