Figure 2. Effects of blockade of endogenous noradrenaline on morphine withdrawal-induced hypersecretion of oxytocin.
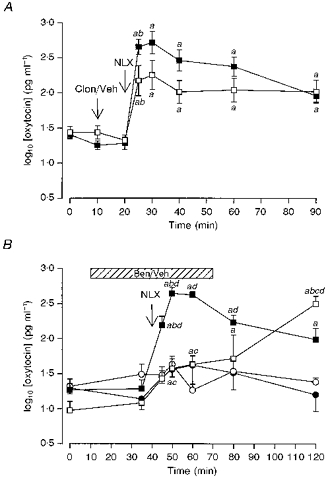
A, plasma oxytocin concentrations (mean log10 plasma oxytocin concentration ±s.e.m.) measured in urethane-anaesthetized morphine-dependent rats. Morphine withdrawal was induced by injection of naloxone (NLX, 5 mg kg−1, i.v.). Rats were given an i.v. injection of either clonidine (Clon; 2.5 mg kg−1; □) or vehicle (Veh; 0.9% saline, ▪) 10 min before injection of naloxone. The increase in oxytocin concentration after naloxone was significantly less in the clonidine-treated rats (P < 0.05); two-way RM ANOVA revealed significant time (P < 0.0001) and interaction (P < 0.01) effects. a, P < 0.05 compared with basal concentration (t = 0 min); b, P < 0.05 compared with the preceding sample. B, plasma oxytocin concentrations (mean log10 plasma oxytocin concentration ±s.e.m.) measured in pentobarbitone-anaesthetized morphine-dependent rats (squares) and morphine-naïve rats (circles). Morphine withdrawal was elicited acutely by injection of naloxone (NLX, 5 mg kg−1, i.v.). Rats were given an i.c.v. infusion (hatched bar) of either benoxathian (5.3 μg min−1, i.c.v. at 0.53 μl min−1, open symbols) or vehicle (0.9% saline, closed symbols). Naloxone evoked an increased release of oxytocin in all groups (n = 6–8), but the magnitude of release was much greater in morphine-dependent rats. Benoxathian infusion attenuated and delayed the naloxone-induced secretion of oxytocin in dependent rats. Two-way ANOVA revealed a significant effect of morphine pretreatment (P < 0.0001) and a significant interaction between the effects of morphine pretreatment and benoxathian treatment (P < 0.05). Two-way RM ANOVA between the groups revealed significant group, time and interaction effects (all P < 0.0001). a, P < 0.05 compared with basal concentration (t = 0 min); b, P < 0.05 compared with the preceding sample; c, P < 0.05 compared with the appropriate time-matched, vehicle-treated, morphine-dependent controls; d, P < 0.05 compared with the similarly treated time-matched, morphine-naïve controls (Student-Newman-Keuls post hoc analyses).
