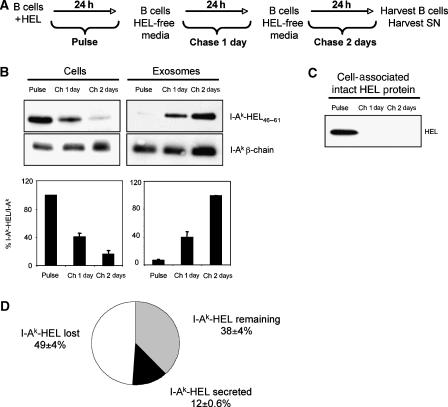
Figure 2.
Appearance of I-Ak-HEL46−61 complexes on exosomes correlates with their disappearance from B cells. (A) To analyze the kinetics of appearance of I-Ak-HEL46−61 complexes on B cells and B cell-derived exosomes, LK35.2 B cells were incubated with 1 mg/ml HEL protein for 24 h before the cell supernatant (and an aliquot of the cells) was harvested (time=Pulse). The remaining cells were washed, replated in HEL/exosome-free medium, and after 1 day the supernatant (and an aliquot of the cells) was harvested (time=Chase 1 day). This process was repeated at 2 days post-pulse (time=Chase 2 day) and exosomes were purified by differential centrifugation. In each condition, the exosomes were concentrated 10-fold relative to the volume of the cell lysate. (B, C) Equal amounts of B cell lysate (B, C) or B cell-derived exosomes (B) were analyzed by immunoblot analysis for intact HEL protein, I-Ak-HEL46−61 complexes (using mAb C4H3), or total I-Ak (using a rabbit anti-I-Ak β-chain serum). The intensity of each band was determined by densitometry and the ratio of I-Ak-HEL46−61 complexes to total I-Ak β-chain was calculated for each time point (in arbitrary units). This value was expressed relative to the value of the Pulse sample (for cells), and to the value of the Chase 2 day sample (for exosomes). (D) The total recovery of I-Ak-HEL46−61 complexes from B cells between the Pulse and the Chase 1 day time point was quantified to be only 38%, and during this time 12% of the initial amount of I-Ak-HEL46−61 complexes present on the cell was released on exosomes. The data represent quantitative analysis of data (average±s.d.) obtained from three independent experiments.