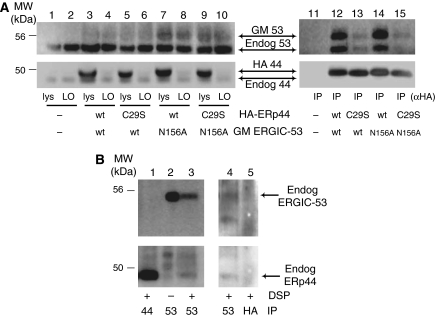
Figure 3.
ERp44 interacts with ERGIC-53. (A) After crosslinking with DSP, aliquots (100 μg of protein) of the RIPA lysates from HeLa transfectants expressing various combinations of wt or mutant HA-ERp44 and GM ERGIC-53 as indicated were IP with anti-HA antibodies and resolved by reducing SDS–PAGE (lanes 11–15). Smaller aliquots of the lysates (30 μg), before (lys) and after IP (left overs (LO), lanes 1–10), were loaded to verify the levels of expression and immunoprecipitation efficiency. After transferring to nitrocellulose, blots were decorated with polyclonal anti-ERGIC-53 (upper panels) and anti-ERp44 (lower panels). Both antibodies recognize doublets: the upper band corresponds to the tagged exogenous molecules, and the faster migrating one to the endogenous ones. The diffuse appearance of exogenous GM ERGIC-53 is due to the presence of N-glycans. Note that anti-HA antibodies efficiently precipitate exogenous, but not endogenous ERp44 molecules (lanes 3–10). In cells overexpressing wt ERp44 (lanes 12 and 14), abundant exogenous and endogenous ERGIC-53 co-IP with ERp44. Co-immunoprecipitation is less efficient in cells expressing the inactive ERp44 C29S mutant (lanes 13 and 15). In contrast, mutation in ERGIC-53 active site (N156A) has only minor effects (lane 14). When inactive mutants of both ERp44 and ERGIC-53 (C29S and N156A, respectively) are coexpressed, some ERGIC-53 can still be co-IP with ERp44, suggesting the two molecules can directly, but transiently, interact. ERGIC-53 did not co-immunoprecipitate in non-transfected cells (lane 11). (B) Endogenous ERp44 and ERGIC-53 non-covalently interact. HeLa cells (300 μg), treated with or without DSP as indicated and lysed in RIPA buffer supplemented with 2 mM Ca2+, were IP with anti-ERGIC-53 (lanes 2 and 3), or anti-ERp44 monoclonal antibodies (lane 1), to confirm the identity of the bands co-IP by anti-ERGIC-53, and resolved by reducing SDS–PAGE. The nitrocellulose was sequentially decorated with rabbit anti-ERGIC-53 (upper panel) and anti-ERp44 antibodies (lower panel), as indicated. Endogenous ERp44 can be co-IP with endogenous ERGIC-53, the association being more evident after crosslinking. In a similar, independent experiment (right panel), anti-HA (lane 5) was used in parallel with anti-ERGIC-53 (lane 4), as a further specificity control for the ERp44 co-IP with anti-ERGIC-53. The blot was first probed with anti-ERp44 and then with anti-ERGIC-53. In this experiment, the ERp44-specific monoclonal antibody did not co-immunoprecipitate detectable ERGIC-53, possibly because of its lower efficiency in immunoprecipitation.