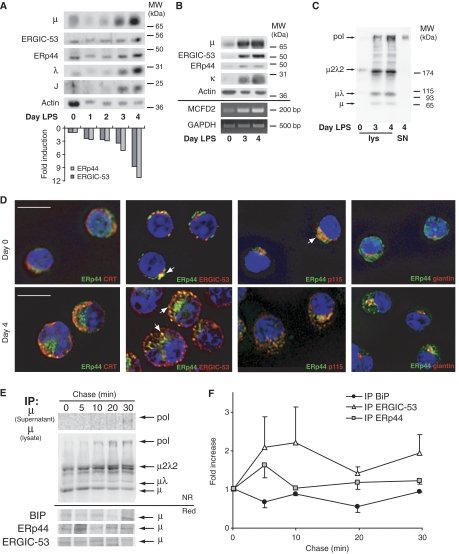
Figure 7.
Localization and role of ERp44 and ERGIC-53 in IgM-secreting B cells. (A–C) Coordinated expression of ERp44 and ERGIC-53 in differentiating B cells, concomitant with the onset of IgM polymerization. I.29μ+ B-lymphoma cells (A) or primary murine splenocytes (B, C) were stimulated in vitro with LPS to induce plasmacytic differentiation. At the indicated days, aliquots were lysed and resolved electrophoretically under reducing (A, B) or NR (C) conditions. An aliquot of the secreted material at day 4 was loaded under NR conditions as a marker of polymers (SN, last lane on the right, panel C). Densitometric quantifications (A, lower panel) were normalized relative to the actin signal. Aliquots of primary B cells at days 0, 3 and 4 of differentiation were also subjected to RNA extraction, RT and PCR to amplify MCFD2 mRNA. GAPDH was used as a control for normalization. Note that ERGIC-53, ERp44 and MCFD2 are simultaneously upregulated during the last stages of B-cell differentiation, concomitantly with J-chain induction and the onset of IgM polymerization. (D) Murine splenocytes at days 0 and 4 of LPS stimulation were stained with the indicated antibodies; images were taken with a fluorescence microscope and analyzed with deconvolution techniques. Arrows indicate examples of colocalizing structures. Not only in B cells (day 0) but also in PCs (day 4 after LPS), ERp44 is located in the ERGIC compartment, showing intense colocalization with ERGIC-53 and p115. Bar=10 μm. (E, F) Dynamic interactions of nascent μ chains with BiP, ERp44 and ERGIC-53. Ramos cells were pulsed for 5 min with 35S-labeled methionine and cysteine, and chased for the indicated times. Culture SN and cell lys were IP with different antibodies as indicated on the left, and then resolved by SDS–PAGE under NR or reducing (red) conditions (E). After transfer to nitrocellulose, blots were subjected to autoradiography. Filters were then decorated with anti-μ antibodies, to verify the identity of the band co-IP with the different interactors (data not shown). Soon after the 5-min pulse, μ, μλ and μ2λ2 are already detectable in the lys. Polymers appear later, being easily detectable after 10 min of chase in the lys, and after 20–30 min in the SN. Densitometric quantifications of radioactive μ chains co-IP with BiP, ERp44 and ERGIC-53 were performed on reduced blots. Signals were normalized relative to a stable background, and data shown as ratio to the signal obtained at time 0 (average of three independent experiments, ±s.e.m.) (F). The level of labeled μ co-IP with BiP decreases immediately after the pulse and is reduced to one-half within 20 min. On the contrary, ERp44- and ERGIC-53-associated μ chains peak at the first chase points, just before IgM polymerization occurs.