Figure 2. Comparison of the single-channel properties of human and murine CFTR.
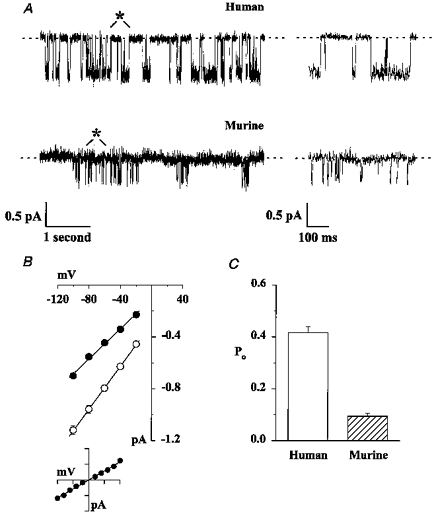
A, representative single-channel records of human and murine CFTR. ATP (1 mM) and the catalytic subunit of PKA (75 nM) were present in the intracellular solution. Voltage was clamped at -50 mV, and there was a large Cl− concentration gradient across the membrane patch (external [Cl−], 10 mM; internal [Cl−], 147 mM). Traces on the left are 5 s long, the 500 ms portions indicated by asterisks are shown on an expanded time scale to the right. B, single-channel I-V relationships of human (○) and murine (•) CFTR. Symbols and error bars indicate means ±s.e.m. of n = 5-6 and n = 7 for human and murine CFTR, respectively, determined using the conditions described in A. Where error bars are not evident the symbol has obscured them. The inset shows the I-V relationship of murine CFTR when the membrane patch was bathed in symmetrical 147 mM Cl− solutions (abscissa: -100 to +100 mV; ordinate: -1 to +1 pA). Data are means ±s.e.m. (n = 5). Under these conditions, reversal potential (Erev) was 2.0 ± 0.7 mV (n = 5) and ECl was 0 mV. Lines indicate the mean slope conductance calculated from the slope conductance of individual experiments. C, Po of human and murine CFTR. Columns and error bars indicate means +s.e.m. (n = 6). Other details as in A. In excised inside-out membrane patches from CHO cells expressing wild-type human CFTR, the single-channel conductance was 9.03 ± 0.18 pS (n = 7) and the Po was 0.43 ± 0.05 (n = 5) under the conditions described in A. These values are not significantly different from those of human CFTR Cl− channels in membrane patches from C127 cells expressing wild-type human CFTR (P > 0.1).
