Figure 2. The conductance activated by ATP was cation selective with Cs+ being 9-fold more permeant than TEA+.
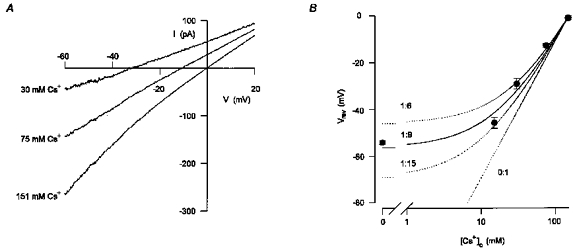
A, mean whole-cell currents activated by extracellular ATP in response to voltage ramps in extracellular solutions containing either 151 mM CsCl or different combinations of CsCl and TEA-Cl. In each extracellular solution, the net current activated by ATP was assessed by subtracting the current recorded under control conditions from the current recorded in the presence of 200 μM ATP. The concentrations of Cs+ in the extracellular solution are indicated by each current trace. The osmolarity of the solution was maintained the same by equimolar substitution of CsCl with TEA-Cl. The extracellular solution also contained (mM): 5 Tes, 10 D-glucose, 1 DAP and 0.1 BaCl2 which was found to be required for the stability of the recordings. Five to eight consecutive current traces were averaged in each experimental condition. Digitized at 1 kHz, filtered at 0.5 kHz. Cell capacitance, 19 pF; series resistance, 5.5 MΩ. B, the reversal potential of the current activated by ATP had a non-linear dependency on the log concentration of Cs+. The mean (±s.e.m.) reversal potentials of the current activated by ATP in extracellular solutions containing different concentrations of Cs+ (symbols) were averaged from 5–15 experiments of the type shown in A. The relative permeability of TEA+ and Cs+(PTEA/PCs) was estimated to be 1:9 from eqn (9) in Results (continuous line). Permeability ratios of 1:6, 1:15 and 0:1 are also shown (dotted lines).
