Figure 1. Experimental determination of pKa of Tyrode solution.
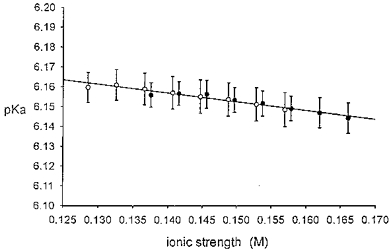
Tyrode solution saturated with 5 % CO2-95 % O2 was titrated with NaHCO3 (4 mM aliquots) at 37 °C, and steady-state pH was measured. Each pH value was used to compute apparent pKa from the Henderson-Hasselbalch equation (see text for details). This value is then plotted versus computed solution ionic strength. •, normal CO2-buffered Tyrode solution composition except for the varied [HCO3−]. ○, as above, but without divalent cation salts CaCl2 and MgCl2. See ‘Solutions’ in Methods for Tyrode solution composition. Line fitted by least-squares linear regression; n= 10 for all samples. No significant difference between • and ○, P > 0.05. Normal Tyrode solution containing 22 mM HCO3−, pH 7.40, has an ionic strength of 0.16 M, at which the graph indicates a pKa of 6.15.
