Figure 9. Modelling results.
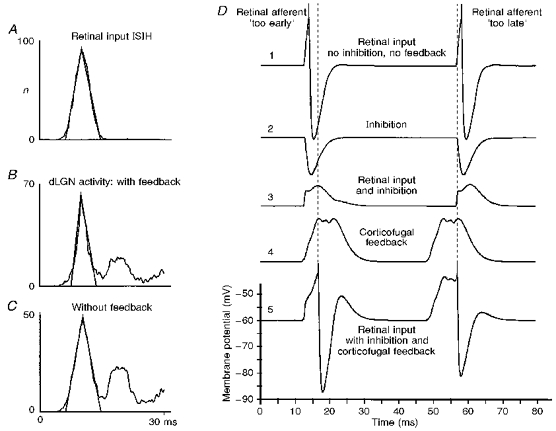
A-C, reduction of the temporal dispersion of dLGN spiking obtained from the computer model. The spatial weighting function for all excitatory projections has the shape of a truncated Gaussian function with a range of 7 cells for thalamo-cortical, 11 cells for the intracortical and 9 cells for the cortico-thalamic projections. Each dLGN relay neuron receives input from 2 inhibitory interneurons, which are also weakly stimulated by the corresponding cortical neurons. A delay was chosen of 3.5 ms for the thalamo-cortical projections, 0.5 ms for intracortical connections and 5.5 ms for corticofugal feedback. A, ISIH of the retinal input, modelled by a truncated Gaussian function of mean, 10 ms and standard deviation, 2 ms. B, corresponding ISIH of the target dLGN cell in the presence of inhibition leading to a partial elimination of spikes and the generation of higher order interval peaks. The corticofugal feedback is active in B. C, ISIH of a dLGN cell in the absence of corticofugal feedback. D, mechanisms of the sharpening effect in a model cell. The top trace (1) shows the action potentials in a model dLGN cell generated by a retinal input that is slightly ahead (left side) or slightly delayed (right side) with respect to the inputs to neighbouring dLGN cells. In the case where the generation of the action potential is masked by inhibition (trace 2) - presumably from thalamic interneurons - the combined input would remain subthreshold (trace 3). However, the corticofugal feedback (trace 4), representing a temporally averaged bulk signal, further modulates the membrane potential of the dLGN cell and leads to a temporal shift of the misplaced action potentials (trace 5) towards the timing represented by the bulk signal (dashed line).
