Abstract
Using whole-cell and cell-attached recording configurations, the role of phosphorylation in leptin activation of ATP-sensitive K+ (KATP) channels was examined in the rat CRI-G1 insulinoma cell line.
Whole-cell current clamp recordings demonstrated that, following dialysis with the non-hydrolysable ATP analogue 5′-adenylylimidodiphosphate (AMP-PNP; 3–5 mM), the leptin-induced hyperpolarization and increase in K+ conductance were completely inhibited.
Under current clamp conditions, application of the broad-spectrum protein kinase inhibitor H-7 (10 μM) had no effect on the resting membrane potential or slope conductance of CRI-G1 insulinoma cells and did not occlude the actions of leptin.
Application of the tyrosine kinase inhibitors genistein (10 μM), tyrphostin B42 (10 μM) and herbimycin A (500 nM) all resulted in activation of KATP channels. In cell-attached recordings, the presence of tyrphostin B42 (10 μM) in the pipette solution activated tolbutamide-sensitive KATP channels in CRI-G1 cells. In contrast, the inactive analogues of genistein and tyrphostin B42 were without effect.
The serine/threonine-specific protein phosphatase inhibitors okadaic acid (50 nM) and cyclosporin A (1 μM) did not prevent or reverse leptin activation of KATP channels. In contrast, whole-cell dialysis with the tyrosine phosphatase inhibitor orthovanadate (500 μM) prevented the actions of both leptin and tyrphostin B42.
In conclusion, leptin activation of KATP channels appears to require inhibition of tyrosine kinases and subsequent dephosphorylation. This process is likely to occur prior to activation of phosphoinositide 3-kinase (PI 3-kinase) as wortmannin prevented activation of KATP channels by tyrphostin B42.
It is well established that protein tyrosine kinases regulate a variety of cellular functions including proliferation, differentiation and signalling processes. Although a number of distinct tyrosine kinases and phosphatases have been identified (Levitzki & Gazit, 1995), the physiological actions and the intracellular targets of these proteins remain unclear. There is, however, increasing evidence that tyrosine kinases and phosphatases can modulate a variety of ion channels by either increasing or decreasing channel activity (Siegelbaum, 1994).
In pancreatic β-cells (Keiffer, Heller, Leech, Holz & Habener, 1997) and the CRI-G1 insulin-secreting cell line (Harvey, McKenna, Herson, Spanswick & Ashford, 1997), leptin, the ob gene product, activates ATP-sensitive potassium (KATP) channels, an action consistent with suppression of insulin secretion. The leptin receptor shows sequence homology with members of the class I cytokine receptor superfamily (Tartaglia et al. 1995), which are thought to signal via association with tyrosine kinases of the janus kinase (JAK) family. Indeed, the long form of the leptin receptor (OB-Rb) activates JAK2 in a haematopoetic cell line (Ghilardi & Skoda, 1997). Several pathways can be activated by JAKs including the insulin receptor substrate proteins (Ihle, 1995). Phosphoinositide 3-kinase (PI 3-kinase) is just one of many proteins associated with the signalling downstream of insulin receptor substrate-1 (IRS-1; Myers & White, 1996). Recently, we have shown that the ability of leptin to activate KATP channels is not only regulated by insulin but also that the pathway underlying this action of leptin involves activation of PI 3-kinase (J. Harvey & M. L. J. Ashford, unpublished observations). Prolactin is also capable of activating JAK2 (Prevarskaya, Skryma, Vacher, Daniel, Djiane & Dufy, 1995) and PI 3-kinase (Berlanga, Gualillo, Buteau, Applanat, Kelly & Edery, 1997), thus the signalling capabilities of the leptin receptor in CRI-G1 cells may show parallels to those of other class I cytokine receptors. Since tyrosine phosphorylation plays a critical role in the actions of other cytokines, we have examined the effects of inhibitors of tyrosine kinases and phosphatases in the present study, in order to elucidate further the mechanism underlying leptin activation of KATP channels in CRI-G1 insulinoma cells.
In addition to protein tyrosine kinases, the activity of ion channels can be modulated by serine/threonine-specific protein kinases (Jonas & Kaczmarek, 1996). Indeed phorbol ester-induced activation of protein kinase C results in phosphorylation and subsequent activation of KATP channels in insulin-secreting cells (Ribalet, Eddlestone & Ciani, 1988; De Weille, Schmid-Antomarchi, Fosset & Lazdunski, 1989). Furthermore, in another insulin-secreting cell line RINm5F (Ribalet, Ciani & Eddlestone, 1989) and rabbit arterial smooth muscle (Quayle, Bonev, Brayden & Nelson, 1994), KATP channel activity is enhanced via protein kinase A-dependent phosphorylation. Consequently we have also examined whether leptin activates KATP channels in CRI-G1 cells via serine/threonine-specific protein kinases. We have reported previously that tyrosine kinase inhibitors mimic leptin activation of KATP channels in CRI-G1 insulin-secreting cells (Ashford & Harvey, 1997).
METHODS
Cell culture
Cells from the rat insulin-secreting cell line CRI-G1 were grown in Dulbecco's modified Eagle's medium with sodium pyruvate and glucose (Life Technologies), supplemented with 10 % fetal calf serum (Sigma) and 1 % penicillin-streptomycin at 37°C in a humidified atmosphere of 95 % air and 5 % CO2. Cells were passaged every 2-5 days as described previously (Carrington, Rubery, Pearson & Hales, 1986), plated onto 3.5 cm Petri dishes (Falcon 3001) and used 1-4 days after plating.
Electrophysiological recording and analysis
Experiments were performed using whole-cell current clamp recording to monitor membrane potential with excursions to voltage clamp mode to examine macroscopic currents and the cell-attached configuration to examine single channel responses, as described previously (Harvey et al. 1997). During voltage clamp recordings, the membrane potential was clamped at −50 mV and 10 mV steps of 100 ms duration were applied every 200 ms (range, −120 to −30 mV). Current and voltage were measured using the Axoclamp 200B amplifier (Axon Instruments) for whole-cell recordings and a List EPC-7 for cell-attached single channel recordings. Currents evoked in response to the voltage step protocol were analysed using pCLAMP 6.0 software (Axon Instruments) whereas current clamp data were recorded onto digital audiotapes and replayed for illustration on a Gould TA 240 chart recorder. Single channel data were analysed for current amplitude and channel activity (NfPo; where Nf is the number of functional channels and Po is the open probability) as described previously (Lee, Rowe & Ashford, 1995). All data are expressed as means ± s.e.m. and statistical analyses were performed using Student's unpaired t test (unless otherwise stated). P < 0.05 was considered significant.
Recording electrodes were pulled from borosilicate glass and had resistances of 1-5 MΩ for whole-cell recordings and 8-12 MΩ for cell-attached experiments when filled with electrolyte solution. The pipette solution for whole-cell recordings comprised (mM): 140 KCl, 0.6 MgCl2, 2.73 CaCl2, 5.0 ATP, 10 EGTA, 10 Hepes, pH 7.2 (free [Ca2+] of 100 nM), whereas for cell-attached single channel recordings the pipette solution contained (mM): 140 KCl, 1 CaCl2, 1 MgCl2, 10 Hepes, pH 7.2. The bath solution was normal saline (mM): 135 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 Hepes, pH 7.4. All solution changes were achieved by superfusing the bath with a gravity-feed system at a rate of 10 ml min−1, which allowed complete bath exchange within 2 min. All experiments were performed at room temperature (22-25°C).
Tyrphostin B42, genistein, daidzein, tyrphostin 1, ATP, 5′-adenylylimidodiphosphate (AMP-PNP), tolbutamide, sodium orthovanadate, herbimycin A and cyclosporin A were all obtained from Sigma. Recombinant human leptin was supplied by Dr Peter Lind of Pharmacia-Upjohn (Stockholm, Sweden). Okadaic acid was obtained from Research Biochemicals International. Tolbutamide was made up as a 100 mM stock solution in DMSO, whereas ATP and AMP-PNP were stored as 100 mM stock solutions in 10 mM Hepes (pH 7.2) and kept at -4°C until required. Genistein, tyrphostin B42, daidzein, herbimycin A and tyrphostin 1 were all made up as 10 mM stock solutions in DMSO. Okadaic acid was made up as a 50 μM stock in DMSO and cyclosporin A was stored as a 1 mM stock in 100 % ethanol.
RESULTS
Leptin activates KATP channels
Under current clamp conditions with 5 mM ATP present in the electrode solution to maintain KATP channels in the closed state, the mean resting membrane potential of CRI-G1 cells was -38 ± 1.7 mV (n = 68). Application of the ob gene product leptin (10 nM) hyperpolarized the CRI-G1 cells to -73 ± 3.1 mV (n = 5; Fig. 1A). Examination of the voltage clamped macroscopic currents indicated that prior to the addition of leptin the slope conductance of the cells was 0.57 ± 0.06 nS and that following exposure to leptin there was an increase in the slope conductance to 7.84 ± 1.23 nS (n = 5; Fig. 1A). The reversal potential (obtained from the point of intersection of the current- voltage relationships) associated with the leptin increase in conductance was -79 ± 1.8 mV (n = 5), which is close to calculated value for the K+ equilibrium potential (EK; −84 mV) in this system, indicating an increase in K+ conductance. The sulphonylurea tolbutamide (100 μM) completely reversed the leptin-induced hyperpolarization and increase in conductance to pre-leptin levels, such that the membrane potential and slope conductance values in the presence of tolbutamide were -41 ± 2.1 mV and 0.61 ± 0.05 nS, respectively (n = 4). Thus these data indicate that leptin activates KATP channels in CRI-G1 insulin-secreting cells, in agreement with previous studies using this cell line (Harvey et al. 1997) and pancreatic β-cells (Keiffer et al. 1997).
Figure 1. Leptin activation of KATP channels is blocked by AMP-PNP.
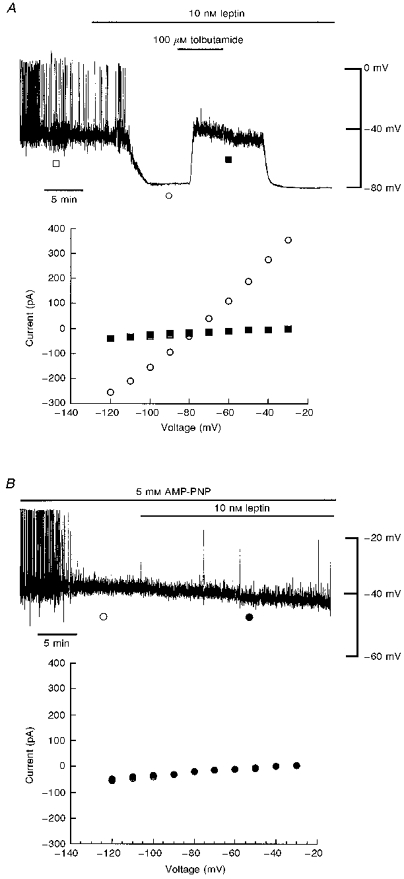
A, top panel: whole-cell current clamp record of a CRI-G1 cell following dialysis with a 5 mM ATP-containing electrode solution. In this and subsequent figures, the trace begins approximately 5 min after obtaining the whole-cell configuration. Application of leptin (10 nM) for the time indicated resulted in hyperpolarization of the membrane from -39 to −80 mV, an action readily reversed by the sulphonylurea tolbutamide (100 μM). Note that there is a delay of around 2 min from the time of drug addition until complete bath exchange. Bottom panel: plot of the current-voltage relationships for voltage clamped currents obtained at the points specified in the top panel: □, control; ○, leptin; ▪, leptin and tolbutamide. Leptin increased the membrane conductance relative to control and tolbutamide (100 μM) reversed the leptin-induced increase in conductance with a reversal potential of −79 mV. B, top panel: current clamp record of a cell dialysed with 5 mM AMP-PNP. Application of leptin (10 nM) for the time indicated, after at least 15-20 min dialysis with AMP-PNP, failed to hyperpolarize CRI-G1 cells. Bottom panel: plot of the current-voltage relationships for the voltage clamped currents in the top panel. Leptin (•) had no effect on the membrane conductance relative to control (○). AMP-PNP prevented leptin activation of KATP channels.
Leptin activation of KATP channels is prevented by AMP-PNP
In order to investigate whether the mechanism underlying leptin activation of KATP channels involves a phosphorylation-dependent process, the effects of AMP-PNP, a non-hydrolysable analogue of ATP, which does not serve as a substrate for protein kinases, were examined. In this series of experiments 5 mM ATP was replaced with AMP-PNP (3–5 mM) in the pipette solution. In current clamp mode under these conditions, the mean resting membrane potential and slope conductance values, 10-15 min after obtaining the whole-cell configuration, were -39 ± 1.7 mV and 0.48 ± 0.05 nS, respectively (n = 7; Fig. 1B). After at least 20 min of recording (to allow complete dialysis of AMP-PNP into the cell interior), leptin (10 nM) was applied to the bath. In contrast to its actions under control conditions (following dialysis with an electrode solution containing 5 mM ATP), leptin had no effect on CRI-G1 insulinoma cells. Thus, the membrane potential and slope conductance values obtained after 15-20 min exposure to leptin were -38 ± 1.9 mV and 0.52 ± 0.06 nS, respectively (n = 7). These data are consistent with leptin activation of KATP channel currents involving a phosphorylation-dependent process.
H-7 does not occlude leptin activation of KATP channels
Since protein kinases are capable of phosphorylating serine/threonine residues of a range of proteins we examined their role in the actions of leptin using the broad-spectrum protein kinase inhibitor H-7 (Hidaka, Watanabe & Kobayashi, 1991). At the concentration (10 μM) examined, H-7 inhibits protein kinases A, C and G (Hidaka et al. 1991). Under current clamp conditions with 5 mM ATP in the pipette solution, application of H-7 (10 μM) for 10-15 min had no effect on the resting membrane potential of CRI-G1 cells; the mean resting membrane potential in the presence of H-7 (10 μM) was -33 ± 2.0 mV (n = 4; Fig. 2A). Examination of the macroscopic currents under voltage clamp conditions indicated that H-7 had no effect on the slope conductance of CRI-G1 cells as the slope conductance values obtained in the absence and presence of H-7 (10 μM) were 0.53 ± 0.07 and 0.55 ± 0.06 nS, respectively (n = 4). Coapplication of leptin (10 nM) and H-7 (10 μM), after 10-15 min exposure to H-7, resulted in hyperpolarization of cells to -68 ± 4.2 mV (n = 4; Fig. 2B). The leptin-induced hyperpolarization was accompanied by an increase in slope conductance to 2.56 ± 0.34 nS and the reversal potential associated with this increase was -79 ± 0.86 mV (n = 4). In parallel control experiments performed in the absence of H-7, application of leptin (10 nM) hyperpolarized CRI-G1 cells to -70 ± 3.8 mV (n = 4) with a concomitant increase in slope conductance from 0.54 ± 0.07 to 2.81 ± 0.26 nS (n = 4). Tolbutamide (100 μM) completely reversed the actions of leptin after exposure to H-7 with a reversal potential of -80 ± 0.94 mV (n = 3). Thus blockade of serine- or threonine-specific protein kinases does not occlude leptin activation of KATP channels in CRI-G1 insulinoma cells.
Figure 2. H-7 does not prevent leptin activation of KATP channels.
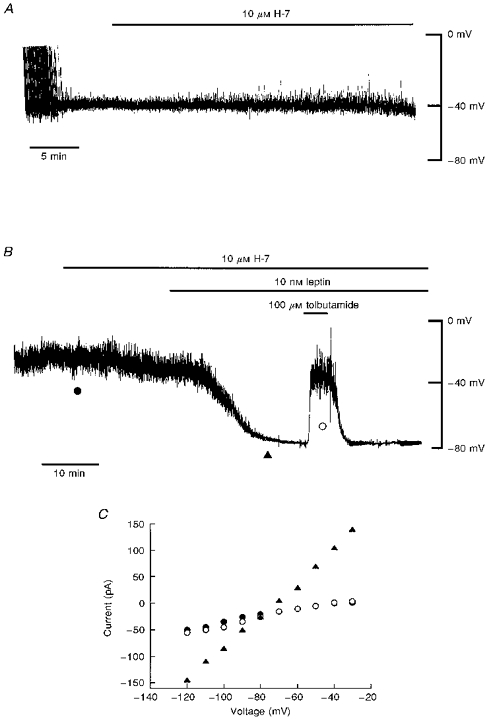
A, current clamp record of a cell dialysed with an electrode solution containing 5 mM ATP. Addition of H-7 (10 μM) for approximately 20 min (as indicated by the bar) had no effect on the resting membrane potential of CRI-G1 cells. B, current clamp record of a cell dialysed with 5 mM ATP-containing electrode solution. Application of H-7 (10 μM) for the time indicated had no effect on the resting membrane potential. Addition of leptin (10 nM) 10-15 min after prior exposure to H-7 (10 μM) resulted in hyperpolarization of CRI-G1 cells from -40 to −78 mV. Tolbutamide (100 μM) applied after the leptin-induced hyperpolarization caused complete reversal of the membrane potential to pre-leptin levels. C, plot of the current-voltage relationships for the voltage clamped currents obtained at the points specified in B: •, H-7; ▴, leptin and H-7; ○, tolbutamide. H-7 (10 μM) did not prevent the leptin-induced increase in K+ conductance.
Tyrosine kinase inhibitors mimic the actions of leptin
In order to determine whether tyrosine phosphorylation is involved in the actions of leptin, the effects of three different tyrosine kinase inhibitors were investigated. Genistein and tyrphostin B42 (Levitzki & Gazit, 1995) are both broad-spectrum tyrosine kinase inhibitors that act via reversible competitive inhibition of ATP and non-competitive inhibition of the protein substrate. In contrast, herbimycin A (Levitzki & Gazit, 1995) inhibits a broad range of tyrosine kinases via competitive inhibition of the protein substrate in an irreversible manner. Under current clamp conditions with 5 mM ATP in the electrode solution to maintain KATP channels in the closed state, application of tyrphostin B42 (10 μM), genistein (10 μM) or herbimycin A (500 nM) resulted in hyperpolarization of the membrane from -39 ± 1.0 mV (n = 24) to -73 ± 1.7 mV (n = 10), -58 ± 3.1 mV (n = 8) and -71 ± 2.6 mV (n = 6), respectively (Fig. 3B and D). Under voltage clamp conditions examination of the macroscopic currents indicated that the hyperpolarization induced by these agents was accompanied by an increase in the slope conductance from 0.53 ± 0.06 to 5.27 ± 0.92 nS (n = 10, tyrphostin B42; Fig. 3D), from 0.56 ± 0.06 to 2.4 ± 0.68 nS (n = 8, genistein; Fig. 3B) and from 0.64 ± 0.06 to 6.23 ± 1.63 nS (n = 6, herbimycin A; data not shown). The increase in slope conductance induced by genistein was significantly less (P < 0.05) than the K+ conductance increase induced by either tyrphostin B42 or herbimycin A. The reversal potentials associated with this increase in conductance were -79 ± 0.9 mV (n = 10; tyrphostin B42), -78 ± 0.8 mV (n = 8; genistein) and -78 ± 0.71 mV (n = 6; herbimycin A), consistent with an increase in K+ conductance. In contrast, the inactive tyrphostin analogue tyrphostin 1 (Levitzki & Gazit, 1995) had no effect on CRI-G1 cells, such that the membrane potential and slope conductance values obtained after 15-20 min exposure to tyrphostin 1 (10 μM) were -32 ± 2.4 mV and 0.56 ± 0.09 nS, respectively (n = 3; Fig. 3C). Furthermore, application of daidzein (10 μM), the inactive analogue of genistein (Levitzki & Gazit, 1995), for at least 20 min also failed to affect either the membrane potential or slope conductance of these cells, with values of -39 ± 1.2 mV and 0.68 ± 0.03 nS, respectively (n = 3; Fig. 3A).
Figure 3. Tyrosine kinase inhibitors activate KATP channel currents.
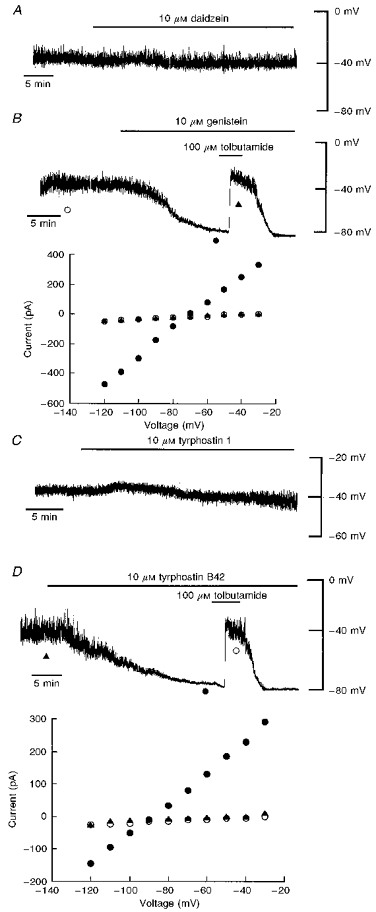
A, current clamp record of a cell dialysed with an electrode solution containing 5 mM ATP. Application of daidzein (10 μM) had no effect on the resting membrane potential of the cell. B, top panel: current clamp trace of a cell dialysed with 5 mM ATP. Addition of genistein (10 μM) resulted in hyperpolarization of the cell membrane to −80 mV. The sulphonylurea tolbutamide (100 μM) completely reversed the genistein-induced hyperpolarization to pre-genistein levels. Bottom panel: plot of the current-voltage relationships for the voltage clamped currents shown at the top: ○, control; •, genistein; ▴, genistein and tolbutamide. The genistein-induced hyperpolarization was accompanied by an increase in K+ conductance. Tolbutamide readily reversed this action of genistein with a reversal potential of −77 mV. C, current clamp record of a CRI-G1 cell dialysed with 5 mM ATP. Application of tyrphostin 1 (10 μM) for at least 25 min failed to affect the resting membrane potential of the cell. D, top panel: current clamp record of a cell dialysed with 5 mM ATP. Application of tyrphostin B42 (10 μM) resulted in hyperpolarization of the CRI-G1 cell to −78 mV. Tolbutamide (100 μM) completely reversed the tyrphostin-induced hyperpolarization to pre-tyrphostin levels. Bottom panel, plot of the current- voltage relationships for the points specified in the top panel: ▴, control; •, tyrphostin B42; ○, tyrphostin B42 and tolbutamide. Tyrphostin B42 increased the membrane conductance relative to control and tolbutamide reversed this action of tyrphostin with a reversal potential of −83 mV.
The sulphonylurea tolbutamide (100 μM) completely reversed the hyperpolarization and increase in slope conductance induced by all three tyrosine kinase inhibitors to pre-drug levels. The reversal potentials associated with this action of tolbutamide were -79 ± 1.1 mV (n = 6, tyrphostin B42; Fig. 3D), -78 ± 1.4 (n = 4, genistein; Fig. 3B) and -78 ± 0.81 mV (n = 6, herbimycin A; data not shown). Together these data indicate that inhibition of tyrosine kinase activity results in activation of KATP channels in CRI-G1 insulin-secreting cells. Cell-attached recordings support these observations and clearly demonstrate that inhibition of tyrosine kinases activates KATP channels (Fig. 4). Application of tyrphostin B42 (10 μM) to the bath during cell-attached recordings (with no tyrphostin B42 in the electrode) did not induce activation of KATP channel currents (n = 7). However, recordings with 10 μM tyrphostin B42 in the electrode solution resulted in activation of KATP channels within 25 min (n = 6) and channel activity was inhibited by application of the sulphonylurea tolbutamide (200 μM; n = 4; Fig. 4B). Analysis of total channel current (over a 120 s period) 2-4 min after formation of the cell-attached configuration resulted in a mean current of 0.26 ± 0.07 pA and this increased to 2.41 ± 0.49 pA after 20-22 min (n = 5; P < 0.05). In contrast, cell-attached recordings with the inactive analogue of tyrphostin B42, tyrphostin 1 (10 μM), in the pipette solution for at least 25 min failed to activate KATP channels (n = 4; Fig. 4A). The mean currents obtained at the same time periods were 0.13 ± 0.04 and 0.14 ± 0.05 pA, respectively (n = 4; P > 0.05).
Figure 4. Activation of single K+ channel currents by tyrosine kinase inhibitors.
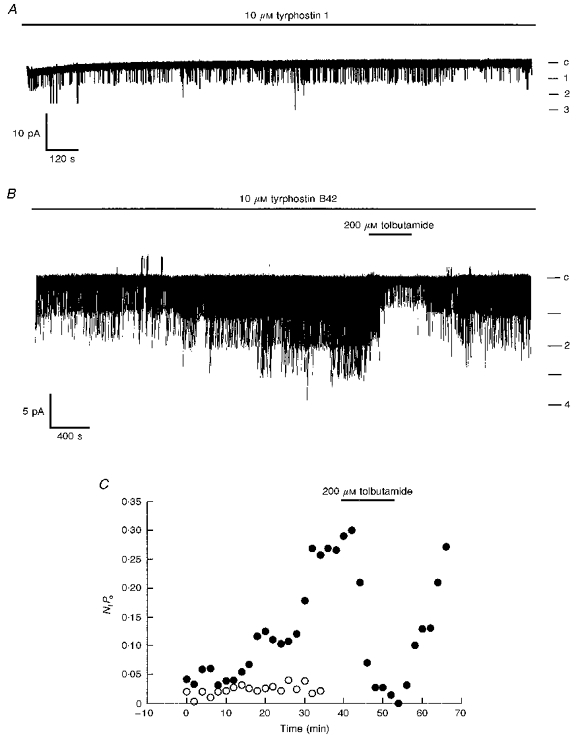
A, cell-attached recording from a CRI-G1 cell, at 10 mV applied to the recording pipette and with 10 μM tyrphostin 1 present in the electrode solution. Continuous recording over a 30 min period showed no significant change in channel activity with time. Note that under these recording conditions channel openings are denoted as downward deflections (inward currents). B, cell-attached recording of a CRI-G1 cell at 10 mV applied potential and with 10 μM tyrphostin B42 in the electrode solution. Single channel activity slowly increased over the time period of recording such that initially two channels appeared to be active and after 15-20 min a maximum of four channels were active. In A and B, c indicates the closed channel level. C, graph of channel activity (NfPo) against time obtained from the cell-attached patches shown in A (○) and B (•).
Application of leptin (10 nM) following the tyrphostin B42-induced hyperpolarization and increase in conductance had no significant effect on the membrane potential or slope conductance (Fig. 5C and D). The mean cell membrane potential was -76 ± 2.3 and -78 ± 2.9 mV (n = 5; P > 0.05) and the slope conductance was 3.9 ± 0.6 and 4.2 ± 0.5 nS (n = 5; P > 0.05) in the absence and presence of leptin, respectively. However, in the presence of genistein (10 μM), which itself hyperpolarized CRI-G1 cells to -52 ± 3.5 mV, leptin (3-10 nM) further hyperpolarized the cells to -80 ± 2.5 mV (n = 4; P > 0.05). This action of leptin was associated with a further increase in conductance from 1.5 ± 0.4 to 6.5 ± 3.4 nS (n = 4) and was completely reversed by tolbutamide to pre-genistein levels (100 μM; n = 4; Fig. 5A). These data indicate that leptin activation of KATP channels is mimicked by three different tyrosine kinase inhibitors and that activation of KATP channels may require dephosphorylation of some unidentified component of the pathway.
Figure 5. The effects of leptin after exposure to tyrosine kinase inhibitors.
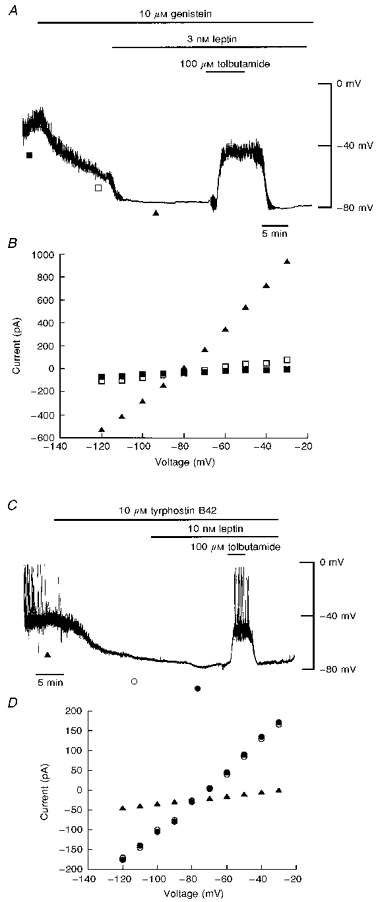
A, current clamp trace of a cell dialysed with 5 mM ATP. Application of genistein (10 μM) hyperpolarized the cell from -38 to −60 mV. Subsequent application of leptin (3 nM) and genistein (10 μM) further hyperpolarized the cell membrane potential to −80 mV. Tolbutamide (100 μM) completely reversed the hyperpolarizations induced by genistein and leptin. B, current-voltage relationships for the voltage clamped currents shown in A: ▪, control; □, genistein; ▴, leptin and genistein. Genistein slightly increased the membrane conductance relative to control. Application of leptin after exposure to genistein resulted in a substantial further increase in K+ conductance. C, current clamp trace of a cell dialysed with 5 mM ATP. Application of tyrphostin B42 (10 μM) hyperpolarized the CRI-G1 cells to −76 mV. Application of leptin (10 nM) after exposure to tyrphostin B42 failed to affect further the cell membrane potential. D, current-voltage relationships for the voltage clamped currents obtained at the times indicated in C: ▴, control; ○, tyrphostin B42; •, leptin and tyrphostin B42. Tyrphostin B42 increased the membrane conductance relative to control, with a reversal potential of −79 mV. Subsequent application of leptin in the presence of tyrphostin B42 failed to increase the membrane conductance.
Serine/threonine-specific phosphatase inhibitors failed to prevent or reverse leptin activation of KATP channels
Since inhibition of tyrosine phosphorylation appears to be required for activation of KATP channels, the next series of experiments investigated whether the effects of leptin could be prevented or reversed by inhibition of dephosphorylation using protein phosphatase inhibitors. Two different serine/threonine-specific protein phosphatase inhibitors were examined, which show specificity for distinct phosphatases; okadaic acid is a potent inhibitor of protein phosphatases 1 and 2A (PP1 and PP2A; Haystead et al. 1989) whereas cyclosporin A is a membrane-impermeant inhibitor of protein phosphatase 2B (PP2B; Liu, Farmer, Lane, Friedman, Weissman & Schreiber, 1991).
In order to determine whether inhibition of PP1 and PP2A prevents leptin activation of KATP channels, cells were exposed to okadaic acid prior to the addition of leptin. In four cells dialysed with an electrode solution containing 5 mM ATP, application of okadaic acid (10-50 nM) for 15-20 min had no effect on the resting membrane potential (-38 ± 2.1 mV; n = 4) and slope conductance (0.38 ± 0.06 nS; n = 4; Fig. 6A). Addition of leptin (10 nM) after 20-30 min exposure to okadaic acid resulted in hyperpolarization of CRI-G1 cells to -70 ± 2.3 mV with an associated increase in slope conductance to 8.63 ± 1.1 nS (n = 4; Fig. 6A). This action of leptin was due to activation of KATP channels as the sulphonylurea tolbutamide (100 μM) completely reversed the actions of leptin with a reversal potential of -77 ± 1.8 mV (n = 4; Fig. 6A). Thus these data suggest that the ability of leptin to activate KATP channels is not prevented by inhibition of PP1 and PP2A.
Figure 6. Serine/threonine-specific phosphatase inhibitors do not prevent the actions of leptin.
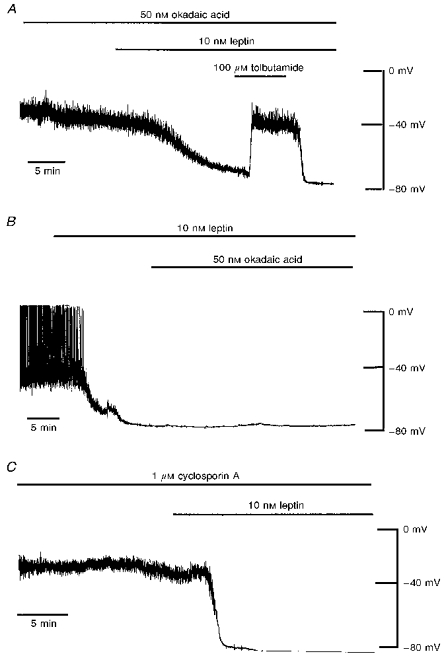
A, current clamp trace of a cell dialysed with 5 mM ATP. Application of okadaic acid (50 nM) for 15-20 min had no effect on the resting membrane potential of CRI-G1 cells. Addition of leptin (10 nM) after prior exposure to okadaic acid resulted in hyperpolarization of CRI-G1 cells to −78 mV. Tolbutamide (100 μM) reversed the leptin-induced hyperpolarization to control levels. B, current clamp record of another cell dialysed with 5 mM ATP. Leptin (10 nM) applied for the time indicated hyperpolarized the cell membrane from -41 to −77 mV. Okadaic acid (50 nM) was unable to reverse the activation of KATP channels by leptin. C, current clamp record of a cell dialysed with 5 mM ATP and 1 μM cyclosporin A. Dialysis of cells with cyclosporin A (1 μM) for at least 15 min had no effect on the resting membrane potential and did not prevent the leptin (10 nM)-induced hyperpolarization.
Similarly, bath application of okadaic acid (50 nM) following the addition of leptin was unable to reverse leptin activation of KATP channels. In cells dialysed with 5 mM ATP, application of leptin (10 nM) hyperpolarized CRI-G1 cells from -41 ± 1.9 to -71 ± 4.8 mV (Fig. 6B) with a concomitant increase in slope conductance from 0.47 ± 0.06 to 6.91 ± 1.6 nS (n = 5). However, following activation of KATP channels by leptin (10 nM), coapplication of okadaic acid (50 nM) and leptin (10 nM) for at least 30 min had no significant effect on the membrane potential (-72 ± 4.5 mV) or slope conductance (6.93 ± 1.7 nS) values (n = 5; P > 0.05; Student's paired t test; Fig. 6B). Thus these data indicate that blockade of PP1 and PP2A with okadaic acid neither occludes nor reverses the actions of leptin.
In another series of experiments the effects of the PP2B inhibitor cyclosporin A on leptin activation of KATP channels were examined. After obtaining the whole-cell configuration and following 15 min dialysis with an electrode solution containing 5 mM ATP and 1 μM cyclosporin A, the mean resting membrane potential and slope conductance of CRI-G1 cells were -38 ± 1.6 mV and 0.47 ± 0.1 nS, respectively (n = 4). Subsequent application of leptin (10 nM) resulted in hyperpolarization of CRI-G1 cells to -68 ± 4.3 mV with an associated increase in slope conductance to 4.71 ± 0.79 nS (n = 4; Fig. 6C). The reversal potential associated with this increase in conductance was -80 ± 1.3 mV, indicating an increase in K+ conductance (n = 4). Together these data indicate that inhibition of dephosphorylation by serine/threonine-specific protein phosphatases does not prevent leptin activation of KATP channels.
Inhibition of tyrosine phosphatases prevents leptin activation of KATP channels
Since serine/threonine-specific phosphatases do not prevent the actions of leptin, the effects of the broad-spectrum protein tyrosine phosphatase inhibitor orthovanadate (Swarup, Cohen & Garbers, 1982) were examined. In order to determine whether inhibition of dephosphorylation by tyrosine phosphatases affects leptin activation of KATP channels, the cells were dialysed with an electrode solution containing 5 mM ATP and 500 μM orthovanadate. Under these conditions, the mean resting membrane potential and slope conductance of CRI-G1 cells were -42 ± 1.4 mV and 0.60 ± 0.07 nS, respectively (n = 5). Application of leptin (10 nM) following dialysis for at least 20 min failed to result in membrane hyperpolarization or an increase in the slope conductance in four of the five cells examined (Fig. 7). Thus the membrane potential and slope conductance values obtained 20 min after leptin application were -46 ± 4.7 mV and 0.98 ± 0.32 nS, respectively (n = 5; P > 0.05; Fig. 7). In another three cells, dialysis with the tyrosine phosphatase inhibitor orthovanadate (500 μM) prevented the tyrphostin B42-induced hyperpolarization and increased K+ conductance (data not illustrated), indicating that the effects of the tyrosine kinase inhibitors on KATP channels are also ultimately due to dephosphorylation. Together these data indicate that inhibition of tyrosine phosphatases by orthovanadate and therefore inhibition of dephosphorylation prevents leptin and tyrphostin B42 activation of KATP channels.
Figure 7. Orthovanadate prevents leptin activation of KATP channels.
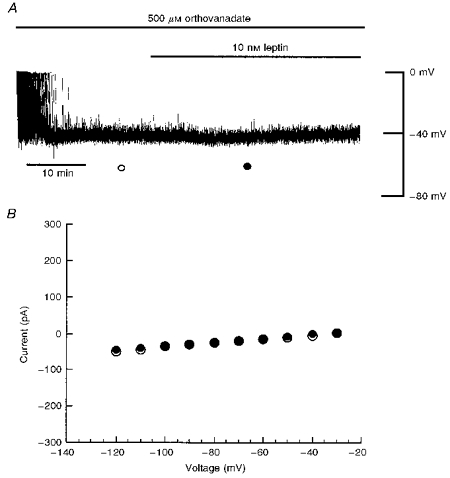
A, current clamp record of a cell dialysed with an electrode solution containing 5 mM ATP and 500 μM orthovanadate. Dialysis with orthovanadate had no effect on the resting membrane potential of CRI-G1 cells; however, it did prevent the leptin-induced hyperpolarization. B, plot of current-voltage relationships for voltage clamped macroscopic currents obtained at the times specified in A: ○, orthovanadate; •, orthovanadate and leptin. Orthovanadate blocked the leptin-induced increase in K+ conductance.
Wortmannin occludes the actions of tyrphostin B42
We have shown previously that leptin activates KATP channels in CRI-G1 cells via a PI 3-kinase-dependent process, as wortmannin, a specific inhibitor of PI 3-kinase (Powis et al. 1994), inhibits the actions of leptin (J. Harvey & M. L. J. Ashford, unpublished observations). In order to determine whether inhibition of the tyrosine kinases (and therefore tyrosine dephosphorylation) occurs prior to or downstream of PI 3-kinase activation, the effects of wortmannin on tyrphostin B42-induced activation of KATP channels were examined (Fig. 8). Under current clamp conditions with 5 mM ATP in the electrode solution, application of wortmannin (10 nM) to the bath for 15-20 min failed to affect the membrane potential (-38 ± 2.0 mV; n = 4) or slope conductance (0.58 ± 0.08 nS; n = 4). After at least 20 min exposure to the PI 3-kinase inhibitor (10 nM), coapplication of wortmannin (10 nM) and tyrphostin B42 (10 μM) had no effect on the membrane potential (-39 ± 2.3 mV) or slope conductance (0.61 ± 0.07 nS) values (n = 4; P > 0.05; Student's paired t test; Fig. 8). These data indicate that inhibition of tyrosine phosphorylation by the tyrosine kinase inhibitor tyrphostin B42, leading to activation of KATP channels, occurs prior to the activation of PI 3-kinase.
Figure 8. Wortmannin prevents tyrphostin B42 activation of KATP channels.
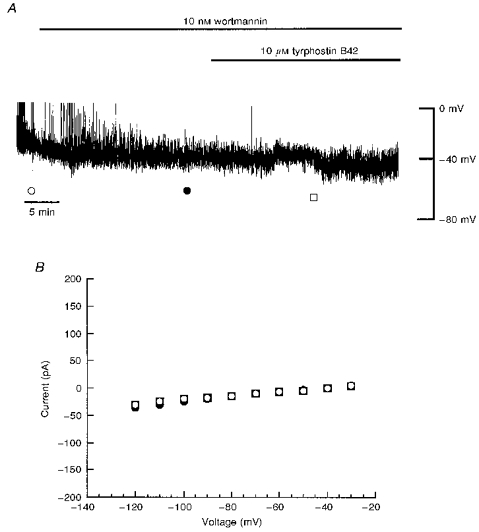
A, current clamp record of a cell dialysed with 5 mM ATP. Application of wortmannin (10 nM) for the time indicated had no effect on the resting membrane potential. Addition of tyrphostin B42 (10 μM) after exposure to wortmannin failed to hyperpolarize CRI-G1 cells. B, plot of the current-voltage relationships for voltage clamped currents obtained at the points specified in A: ○, control; •, wortmannin; □, tyrphostin B42 and wortmannin. Wortmannin prevented the tyrphostin-induced increase in K+ conductance.
DISCUSSION
Recently, we and others have shown that in the CRI-G1 insulin-secreting cell line (Harvey et al. 1997) and pancreatic β-cells (Keiffer et al. 1997) the ob gene product leptin activates KATP channels, an action consistent with suppression of insulin secretion. Peptide modulation of KATP channels has previously been shown to be mediated by protein kinase A (Quayle et al. 1994; Miyoshi & Nakaya, 1995) and protein kinase C (De Weille et al. 1989; Kubo, Quayle & Standen, 1997). Protein kinases are also reported to modulate KATP channel function in insulin-secreting cell lines (Ribalet et al. 1988; Wollheim, Dunne, Peter-Riesch, Bruzzone, Pozzan & Petersen, 1988). In the present study, however, the broad-spectrum protein kinase inhibitor H-7 and the protein phosphatase inhibitors okadaic acid and cyclosporin A had no effect on KATP channels. Furthermore, these agents failed to block leptin activation of KATP channels suggesting that protein phosphorylation of serine/threonine residues does not underlie leptin actions in CRI-G1 cells. As the leptin receptor is a member of the class I cytokine receptor family (Tartaglia et al. 1995), one likely mechanism by which KATP channel activity is increased is via activation of tyrosine kinases such as the JAK family (Ihle, 1995). Indeed, the class I cytokine prolactin activates Ca2+-activated K+ channels via activation of JAK2 in Chinese hamster ovary (CHO) cells (Pervarskaya et al. 1995). In contrast, however, our present data are consistent with leptin activation of KATP channels involving inhibition of tyrosine kinases and subsequent tyrosine dephosphorylation. Thus, dialysis with AMP-PNP, a non-hydrolysable analogue of ATP, prevented leptin activation of KATP channels indicating that phosphorylation or dephosphorylation plays a role in this process. In addition, inhibition of tyrosine kinase activity by application of three different tyrosine kinase inhibitors mimicked the leptin-induced hyperpolarization and increase in K+ conductance. In contrast, the inactive analogues of genistein (daidzein) and tyrphostin B42 (tyrphostin 1) failed to affect the cell membrane potential or slope conductance of CRI-G1 cells. Furthermore, activation of KATP channels by tyrphostin B42 prevented the actions of leptin suggesting that the mechanism underlying leptin activation of KATP channels may involve inhibition of tyrosine kinases (and subsequent tyrosine dephosphorylation). Alternatively, in a manner similar to non-selective cation channels in Aplysia bag cell neurones (Wilson & Kaczmarek, 1993) and leech mechanosensitive neurones (Catarsi & Drapeau, 1997), the actions of leptin in this study may involve the activation of tyrosine phosphatases. This is further supported by the prevention of leptin activation of KATP channels and subsequent hyperpolarization following dialysis with the tyrosine phosphatase inhibitor orthovanadate.
In the present study, tyrphostin B42 and herbimycin A were more effective than genistein in activating KATP channels as both these agents hyperpolarized CRI-G1 cells to more negative potentials and produced a greater increase in K+ conductance than genistein. Furthermore, genistein, in contrast to tyrphostin B42, did not completely occlude leptin activation of KATP channels. This is most probably attributable to differences in the specificity or potency of these inhibitors for distinct tyrosine kinases present in these cells. Further studies are required to elucidate the identity of the tyrosine kinase(s) and phosphatase(s) responsible for modulation of this pathway.
In cell-attached recordings, KATP channels were opened by tyrphostin B42 only when this agent was present in the electrode solution. Similarly, leptin opened KATP channels in the cell-attached configuration when the protein was in direct contact with the extracellular surface of the membrane patch (Harvey et al. 1997). Thus these data suggest that leptin and tyrphostin B42 open KATP channels in a membrane-delimited manner. In a manner similar to leptin (J. Harvey & M. L. J. Ashford, unpublished observations), the effects of tyrphostin B42 were blocked by the PI 3-kinase inhibitor wortmannin, suggesting that the mechanism underlying leptin activation of KATP channels in the CRI-G1 insulin-secreting cell line involves tyrosine dephosphorylation of unidentified cytosolic proteins prior to activation of PI 3-kinase. Recent studies indicate that the insulin receptor substrate proteins, in particular IRS-1, are capable of activating PI 3-kinase following activation of cytokine receptors including insulin (Myers & White, 1996) and prolactin (Berlanga et al. 1997). Furthermore, leptin has been shown to downregulate tyrosine phosphorylation of IRS-1 by insulin in hepatocytes (Cohen, Novick & Rubinstein, 1996). Thus, IRS-1 is one possible target site for dephosphorylation by leptin in this cell line. However, further biochemical experiments are required to determine the protein(s) that is dephosphorylated following leptin receptor activation.
The modulatory action of inhibitors of tyrosine kinases and phosphatases on KATP channel function observed in the present study parallels the actions of these agents on the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel. Thus genistein activates the CFTR chloride channel in a number of expression systems (Illek, Fischer, Santos, Widdicombe, Machen & Reenstra, 1995; Yang, Cheng, Wang, Price & Hwang, 1997), whereas orthovanadate inhibits genistein-induced channel activation (Illek et al. 1995). Tyrosine kinase inhibitors are also reported to increase the activity of other channel types including Ca2+-activated K+ channels in smooth muscle cells (Xiong, Burnette & Cheung, 1995) and hippocampal neurones (Abdul-Ghani, Valiante, Carlen & Pennefather, 1996) and cation channels in leech pressure-sensitive neurones (Aniksztejn, Catarsi & Drapeau, 1997). In contrast, the activity of voltage-dependent Ca2+ channels is reduced by tyrosine kinase inhibitors in GH3 pituitary cells (Cataldi et al. 1996) and dorsal root ganglion neurones (Fitzgerald & Dolphin, 1997), whereas orthovanadate increases the activity of these channels (Cataldi et al. 1996). Therefore, like numerous other channel types, the activity of KATP channels is influenced by the interplay between protein tyrosine kinase and tyrosine phosphatase activity.
The involvement of tyrosine dephosphorylation in leptin activation of KATP channels in CRI-G1 cells contrasts with the role of tyrosine phosphorylation proposed for prolactin activation of Ca2+-activated K+ channels in CHO cells (Prevarskaya et al. 1995), as activation of JAK2 is implicated in the stimulatory action of prolactin. Since prolactin, like leptin, can activate PI 3-kinase (Berlanga et al. 1997), differences in the cellular signalling pathways downstream of PI 3-kinase may explain the opposing roles of tyrosine phosphorylation underlying the actions of these cytokine receptors. In support of this, different isoforms of PI 3-kinase have been identified (Zvelebil et al. 1996), which may activate separate but interesting pathways. Furthermore, this may explain why insulin prevents leptin activation of KATP channels in CRI-G1 insulinoma cells (J. Harvey & M. L. J. Ashford, unpublished observations), even though both these agents activate PI 3-kinase. Alternatively, the basal phosphorylation state of target proteins (or channels) and the number or type (tyrosine) of possible phosphorylation sites may be important.
Leptin has recently been shown to activate KATP channels in hypothalamic glucose-receptive neurones (Spanswick, Smith, Groppi, Logan & Ashford, 1997), an action that may reflect its antiobesity actions in the whole animal. It will obviously be interesting to determine whether the leptin receptor transduction pathway(s) in the brain is identical to that described here or whether significant alternative mechanisms are present in other cell types. There is increasing evidence that a feedback loop exists between adipose tissue and pancreatic β-cells such that administration of insulin increases leptin expression (Mizuno et al. 1996) and application of leptin reduces plasma levels of insulin (Pelleymounter et al. 1995; Halaas et al. 1995). However, in the obese state this feedback loop is dysfunctional, such that obese individuals show resistance to leptin even under conditions of hyperleptinaemia. In the absence of any defect in leptin production or the leptin receptor, leptin resistance is likely to occur downstream of the receptor. In this respect, the involvement of tyrosine dephosphorylation prior to PI 3-kinase stimulation in leptin activation of KATP channels may be important in understanding the signalling processes underlying these defects.
Acknowledgments
This work was supported by The Wellcome Trust (grant no. 042726). We thank Michelle Buchan for cell culture assistance.
References
- Abdul-Ghani MA, Valiante TA, Carlen PL, Pennefather PS. Tyrosine kinase inhibitors enhance a Ca2+-activated K+ current (IAHP) and reduce IAHP suppression of a metabotropic glutamate receptor agonist in rat dentate granule neurones. The Journal of Physiology. 1996;496:139–144. doi: 10.1113/jphysiol.1996.sp021671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniksztejn L, Catarsi S, Drapeau P. Channel modulation by tyrosine phosphorylation in an identified leech neuron. The Journal of Physiology. 1997;498:135–142. doi: 10.1113/jphysiol.1997.sp021846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford MLJ, Harvey J. Leptin activation of KATP channels is mimicked by tyrosine kinase inhibitors in CRI-G1 insulin secreting cells. British Journal of Pharmacology. 1997;122:56. P. [Google Scholar]
- Berlanga JJ, Gualillo O, Buteau H, Applanat M, Kelly PA, Edery M. Prolactin activates tyrosyl phosphorylation of insulin receptor substrate 1 and phosphatidyl-3-OH kinase. Journal of Biological Chemistry. 1997;272:2050–2052. doi: 10.1074/jbc.272.4.2050. 10.1074/jbc.272.4.2050. [DOI] [PubMed] [Google Scholar]
- Carrington CA, Rubery ED, Pearson EC, Hales CN. Five new insulin-producing cell lines with differing secretory properties. Journal of Endocrinology. 1986;109:193–200. doi: 10.1677/joe.0.1090193. [DOI] [PubMed] [Google Scholar]
- Cataldi M, Taglialatela M, Guerriero S, Amoroso S, Lombardi G, Di Renzo G, Annunziato L. Protein-tyrosine kinases activate while protein-tyrosine phosphatases inhibit L-type calcium channel activity in pituitary GH3 cells. Journal of Biological Chemistry. 1996;271:9441–9446. doi: 10.1074/jbc.271.16.9441. 10.1074/jbc.271.16.9441. [DOI] [PubMed] [Google Scholar]
- Catarsi S, Drapeau P. Requirement for tyrosine phosphatase during serotonergic neuromodulation by protein kinase C. Journal of Neuroscience. 1997;17:5792–5797. doi: 10.1523/JNEUROSCI.17-15-05792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B, Novick D, Rubinstein M. Modulation of insulin activities by leptin. Science. 1996;274:1185–1188. doi: 10.1126/science.274.5290.1185. [DOI] [PubMed] [Google Scholar]
- De Weille JR, Schmid-Antomarchi H, Fosset M, Lazdunski M. Regulation of ATP-sensitive K+ channels in insulinoma cells: activation by somatostatin and protein kinase C and the role of cAMP. Proceedings of the National Academy of Sciences of the USA. 1989;86:2971–2975. doi: 10.1073/pnas.86.8.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EM, Dolphin AC. Regulation of rat neuronal voltage-dependent calcium channels by endogenous p21-ras. European Journal of Neuroscience. 1997;9:1252–1261. doi: 10.1111/j.1460-9568.1997.tb01480.x. [DOI] [PubMed] [Google Scholar]
- Ghilardi N, Skoda RC. The leptin receptor activates janus kinase 2 and signals for proliferation in a factor-dependent cell line. Molecular Endocrinology. 1997;11:393–399. doi: 10.1210/mend.11.4.9907. 10.1210/me.11.4.393. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallione RL, Burley SK, Freidman JM. Weight reducing effects of the plasma-protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Harvey J, McKenna F, Herson PS, Spanswick D, Ashford MLJ. Leptin activates ATP-sensitive potassium channels in the rat insulin-secreting cell line, CRI-G1. The Journal of Physiology. 1997;504:527–535. doi: 10.1111/j.1469-7793.1997.527bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haystead TAJ, Sim ATR, Carling D, Honnor RC, Tsukitani Y, Cohen P, Hardie DG. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989;337:78–81. doi: 10.1038/337078a0. 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- Hidaka H, Watanabe M, Kobayashi R. Properties and use of H-series compounds as protein kinase inhibitors. In: Hunter T, Sefton BM, editors. Methods in Enzymology, Protein Phosphorylation, Analysis of Protein Phosphorylation, Protein Kinase Inhibitors and Protein Phosphatases, Protein Kinase Inhibitors. Vol. 201. San Diego, CA, USA: Academic Press Inc.; 1991. pp. 328–339. part B, section II. [DOI] [PubMed] [Google Scholar]
- Ihle JN. Cytokine receptor signalling. Nature. 1995;377:591–594. doi: 10.1038/377591a0. 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- Illek B, Fischer H, Santos GF, Widdicombe JH, Machen TE, Reenstra WM. cAMP-independent activation of CFTR Cl channels by the tyrosine kinase inhibitor genistein. American Journal of Physiology. 1995;268:C886–893. doi: 10.1152/ajpcell.1995.268.4.C886. [DOI] [PubMed] [Google Scholar]
- Jonas EA, Kaczmarek LK. Regulation of potassium channels by protein kinases. Current Opinion in Neurobiology. 1996;6:318–323. doi: 10.1016/s0959-4388(96)80114-0. 10.1016/S0959-4388(96)80114-0. [DOI] [PubMed] [Google Scholar]
- Keiffer TJ, Heller RS, Leech CA, Holz GG, Habener JF. Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic beta cells. Diabetes. 1997;46:1087–1093. doi: 10.2337/diab.46.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M, Quayle JM, Standen NB. Angiotensin II inhibition of ATP-sensitive K+ currents in rat arterial smooth muscle cells through protein kinase C. The Journal of Physiology. 1997;503:489–496. doi: 10.1111/j.1469-7793.1997.489bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Rowe ICM, Ashford MLJ. Characterization of an ATP-modulated large conductance Ca2+-activated K+ channel present in rat cortical neurones. The Journal of Physiology. 1995;488:319–337. doi: 10.1113/jphysiol.1995.sp020969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A, Gazit A. Tyrosine kinase inhibition: An approach to drug developement. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin-A and FKBP and FK506 complexes. Cell. 1991;66:807–812. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Nakaya Y. Calcitonin gene-related peptide activates the K+ channels of vascular smooth muscle cells via adenylate cyclase. Basic Research in Cardiology. 1995;90:332–336. doi: 10.1007/BF00797911. [DOI] [PubMed] [Google Scholar]
- Mizuno TM, Bergen H, Funabashi T, Kleopoulos SP, Zhong YG, Bauman WA, Mobbs CR. Obese gene expression-reduction by fasting and stimulation by insulin and glucose in lean mice, and persistent elevation in acquired (diet-induced) and genetic (yellow agouti) obesity. Proceedings of the National Academy of Sciences of the USA. 1996;93:3434–3438. doi: 10.1073/pnas.93.8.3434. 10.1073/pnas.93.8.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, White MF. Insulin signal transduction and the IRS proteins. Annual Review of Pharmacology and Toxicology. 1996;36:615–658. doi: 10.1146/annurev.pa.36.040196.003151. 10.1146/annurev.pa.36.040196.003151. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Pervarskaya NB, Skryma RN, Vacher P, Daniel N, Djiane J, Dufy B. Role of tyrosine phosphorylation in potassium channel activation. Functional association with prolactin receptor and JAK2 tyrosine kinase. Journal of Biological Chemistry. 1995;270:24292–24299. doi: 10.1074/jbc.270.41.24292. 10.1074/jbc.270.41.24292. [DOI] [PubMed] [Google Scholar]
- Powis G, Bonjouklion R, Berggren MM, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter WF, Dodge J, Grindlay G, Vlahos CJ. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Research. 1994;54:2419–2423. [PubMed] [Google Scholar]
- Quayle JM, Bonev AD, Brayden JE, Nelson MT. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. The Journal of Physiology. 1994;475:9–13. doi: 10.1113/jphysiol.1994.sp020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribalet B, Ciani S, Eddlestone GT. ATP mediates both activation and inhibition of K(ATP) channel activity via cAMP-dependent protein kinase in insulin-secreting cell lines. Journal of General Physiology. 1989;94:693–717. doi: 10.1085/jgp.94.4.693. 10.1085/jgp.94.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribalet B, Eddlestone GT, Ciani S. Metabolic regulation of the K(ATP) and a maxi-K(V) channel in the insulin-secreting RINm5F cell. Journal of General Physiology. 1988;92:219–237. doi: 10.1085/jgp.92.2.219. 10.1085/jgp.92.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. Ion channel control by tyrosine phosphorylation. Current Opinion in Neurobiology. 1994;4:242–246. doi: 10.1016/s0960-9822(00)00054-3. [DOI] [PubMed] [Google Scholar]
- Spanswick D, Smith M, Groppi V, Logan SD, Ashford MLJ. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–525. doi: 10.1038/37379. 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- Swarup G, Cohen S, Garbers DL. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochemical and Biophysical Research Communications. 1982;107:1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Demski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Woolfe EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Wilson GF, Kaczmarek LK. Mode-switching of a voltage-gated cation channel is mediated by a protein kinase A-regulated tyrosine phosphatase. Nature. 1993;366:433–439. doi: 10.1038/366433a0. 10.1038/366433a0. [DOI] [PubMed] [Google Scholar]
- Wollheim CB, Dunne MJ, Peter-Riesch B, Bruzzone R, Pozzan T, Petersen OH. Activators of protein kinase C depolarise insulin-secreting cells by closing K+ channels. EMBO Journal. 1988;7:2443–2449. doi: 10.1002/j.1460-2075.1988.tb03090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z, Burnette E, Cheung DW. Modulation of Ca2+-activated K+ channel activity by tyrosine kinase inhibitors in vascular smooth muscle cell. European Journal of Pharmacology. 1995;290:117–123. doi: 10.1016/0922-4106(95)90023-3. 10.1016/0922-4106(95)90023-3. [DOI] [PubMed] [Google Scholar]
- Yang IC-H, Cheng T-H, Wang F, Price EM, Hwang T-C. Modulation of CFTR chloride channels by calyculin A and genistein. American Journal of Physiology. 1997;272:C142–155. doi: 10.1152/ajpcell.1997.272.1.C142. [DOI] [PubMed] [Google Scholar]
- Zvelebil MJ, MacDougall L, Leevers S, Volinia S, Vanaesebroeck B, Gout I, Panayotou G, Domin J, Stein R, Pages F. Structural and functional diversity of phosphoinositide 3-kinase. Philosophical Transactions of the Royal Society. 1996;B 351:217–223. doi: 10.1098/rstb.1996.0019. [DOI] [PubMed] [Google Scholar]


