Abstract
The present study was designed to examine the interaction between sympathetic and parasympathetic influences on blood flow in oro-facial areas such as lower lip, palate and submandibular gland (SMG) and in the common carotid artery (CCA) in anaesthetized cats.
Section of the ipsilateral superior cervical sympathetic trunk (CST) increased the basal CCA blood flow significantly. The control level with the nerve intact was comparable with that seen at 0.5-1 Hz CST stimulation, suggesting a spontaneous discharge of around 0.5-1 Hz in the CST fibres innervating the beds supplied by the CCA. The basal blood flow at all sites examined was reduced by CST stimulation in a frequency-dependent manner.
Electrical stimulation of the central end of the lingual nerve (LN) evoked blood flow increases in the lower lip and palate. These blood flow increases were markedly reduced by concurrent CST stimulation in a manner that was frequency dependent, but not simply related to the vasoconstrictor effect of CST stimulation. This effect of CST stimulation was not observed in tongue or SMG, even though CST stimulation evoked vasoconstriction in these tissues. A significant reduction in the level of CCA blood flow attained during LN stimulation was observed on repetitive CST stimulation only at 10 Hz, indicating that this response behaved in a fashion different from that seen in the lower lip, palate, tongue and SMG.
The present study suggests that concurrent repetitive CST stimulation reduces parasympathetically mediated blood flow increases in certain oro-facial areas (such as the lower lip and palate), but not in the tongue and SMG. This inhibitory action was not a simple additive effect (between vasoconstriction and vasodilatation) and it disappeared rapidly after the cessation of CST stimulation.
There are several reports in the literature concerning interactions between sympathetic and parasympathetic effects on the heart, nasal mucosa, lower lip and submandibular gland (SMG) (Potter, 1985; Gardner & Potter, 1988; Hall et al. 1990; Revington et al. 1990; Moriarty et al. 1992; Ulman et al. 1992; Ulman et al. 1993; Izumi, 1995; Izumi & Karita, 1995; Karita & Izumi, 1995), and between the effects of sympathetic and sensory nerves on the dental pulp (Kerezoudis et al. 1993; Olgart & Kerezoudis, 1994) and hairless skin (Habler et al. 1997).
Recent studies using cats and dogs have suggested that substances other than noradrenaline (such as neuropeptide Y or galanin) are released during a 3 min period of stimulation of the sympathetic nerves, and that these substances attenuate not only the vagal effect on the heart, but also the parasympathetically evoked vasodilatation in the nasal mucosa (Lacroix et al. 1994a, b). However, such sympathetically mediated attenuation seems to differ from animal to animal and it is not known whether all vascular beds in the oro-facial area are affected in a similar way. Consequently, we decided to carry out a more extensive examination of the interaction between sympathetic effects and parasympathetically evoked vasodilatation in the oro-facial areas of cats. For these experiments, we used a ‘pseudo-reflex’ method (electical stimulation of the central cut end of the lingual nerve (LN)) to activate parasympathetic efferents. We chose this method because we believe that it mimics processes occurring during physiological forms of vasodilatation. Although the stimulus used is clearly not the same as an adequate (‘physiological’) stimulus to the receptors, it is a well established and useful way of evoking parasympathetic vasodilatation while avoiding the possibility of evoking antidromic or sympathetic vasodilatation along with the desired parasympathetic response, as reported previously (Izumi & Karita, 1992, 1994, 1995).
METHODS
Animals
Eighteen adult cats, unselected as to sex and weighing 3.2-4.3 kg, were first sedated with ketamine hydrochloride (30 mg kg−1, i.m.) and then anaesthetized with a mixture of α-chloralose (50 mg kg−1, i.v.) and urethane (100 mg kg−1, i.v.). Supplementary doses were given when necessary throughout the experiment. One cephalic vein was cannulated to allow drug administration and one femoral artery was cannulated and connected to a Statham pressure transducer to monitor systemic arterial blood pressure and heart rate. The anaesthetized animals were intubated, paralysed by intravenous injection of pancuronium bromide (Mioblock, Organon Teknika, The Netherlands; 0.4 mg kg−1 initially, and supplemented with 0.2 mg kg−1 approximately every 60 min after the level of anaesthesia had been tested; see below) and artificially ventilated via the tracheal cannula with a mixture of 50% air-50% O2. The ventilator (Shinano, model SN-480-6, Tokyo, Japan) was set to deliver a tidal volume of 10-12 cm3 kg−1 at a rate of 20 breaths min−1. The end-tidal concentration of inhalation anaesthetics and CO2 was determined by means of an infrared analyser (Capnomac Ultima, Datex Co., Helsinki, Finland). Continuous ventilation in this manner maintained arterial blood pH at 7.4 ± 0.2, arterial CO2 pressure (Pa,CO2) at 35.8 ± 5.8 mmHg and arterial O2 pressure (Pa,O2) at 287.7 ± 33.8 mmHg. Arterial blood was collected from the femoral artery approximately every 1.5 h for measurement of pH, Pa,CO2 and Pa,O2. Ringer solution (Otsuka Pharmaceutical Co., Tokyo, Japan) was continuously infused at a rate of approximately 8 ml h−1, and 8.4 % NaHCO3 solution was added, when necessary, to maintain the arterial blood pH at the value given above. Rectal temperature was maintained at 37-38°C using a heating pad.
The criteria for maintenance of an adequate depth of anaesthesia were the persistence of miotic pupils and the absence of a reflex elevation of heart rate and arterial blood pressure during stimulation of the central end of the LN. If the depth of anaesthesia was considered inadequate, on the basis of the above criteria, additional α-chloralose and urethane (i.e. intermittent doses of 5 and 10 mg kg−1i.v., respectively) were administered. Once an adequate depth of anaesthesia had been attained, supplementary doses of pancuronium were given approximately every 60 min to maintain immobilization during periods of stimulation.
In all experiments, the vagi and sympathetic trunks were cut bilaterally in the neck prior to any stimulation. This avoided the involvement of the cervical sympathetics in any reflex effects, and ensured that only non-vagal parasympathetic effects were involved in the present study. All cats were killed, at the end of the experiment, by an overdose (about 150 mg) of sodium pentobarbitone.
The experimental protocols were reviewed by the Committee on the Ethics of Animal Experiments in the Tohoku University School of Medicine, and carried out in accordance with the Guidelines for Animal Experiments issued by the Tohoku University School of Medicine, and The Law (No. 105) and Notification (No. 6) of the Japanese Government.
Electrical stimulation of the superior cervical sympathetic trunk (CST) and LN
These experiments involved electrical stimulation of the central (a in Fig. 1) or peripheral (b in Fig. 1) cut ends of the LN (at a site some 5 mm distal to the intersection of the LN and the SMG duct), and electrical stimulation of the central cut end of the inferior alveolar nerve (c in Fig. 1) or the peripheral cut end of the CST (d in Fig. 1). For this purpose, all nerves were sectioned and stimulated unilaterally under a binocular microscope. A bipolar silver electrode attached to a Nihon Koden model SEN-7103 stimulator was used for stimulation. The CST was stimulated using a supramaximal voltage (10 V) and 2 ms pulse duration at various frequencies (0.5-10 Hz for 10 min; Figs 2–4). The LN was stimulated for 20 s using a supramaximal voltage (30 V) at 10 Hz with pulses of 2 ms duration either alone or during on-going repetitive CST stimulation. Electrical stimulation of the LN was begun between 2.5 and 7.5 min after the start of the period of repetitive sympathetic stimulation, unless otherwise noted.
Figure 1. Schematic representation of the sites of electrical stimulation.
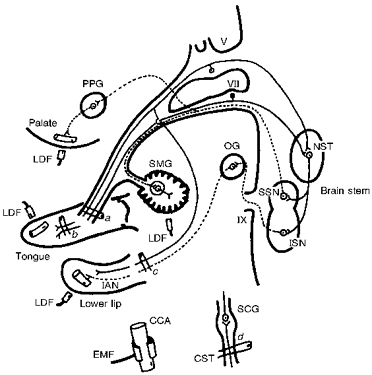
Stimulation sites: central (a) and peripheral (b) cut ends of the lingual nerve, the central cut end of the inferior alveolar nerve (c) and the peripheral cut end of the superior cervical sympathetic trunk (d). The dashed lines indicate the parasympathetic vasodilator fibres from the superior and inferior salivatory nuclei. The continuous lines indicate trigeminal and facial sensory inputs to the brain stem. The sympathetic supply, via the superior cervical ganglion, and the common carotid artery are shown at the bottom. Abbreviations: CCA, common carotid artery; EMF, electromagnetic flowmeter; IAN, inferior alveolar nerve; ISN, inferior salivatory nucleus; LDF, laser-Doppler flowmeter; NST, nucleus of the solitary tract; OG, otic ganglion; PPG, pterygopalatine ganglion; CST, superior cervical sympathetic trunk; SCG, superior cervical ganglion; SMG, submandibular gland; SSN, superior salivatory nucleus; V, trigeminal nerve root; VII, facial nerve root; IX, glossopharyngeal nerve root.
Figure 2. Effects of section of the CST and of electrical stimulation of its peripheral cut end on the basal CCA blood flow level, and of electrical stimulation of the central cut end of the LN on CCA blood flow during a period of repetitive CST stimulation at each stimulation frequency.
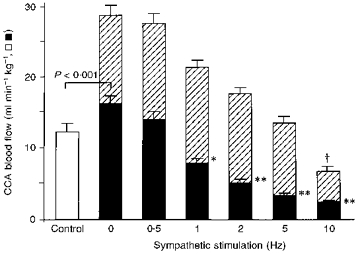
Parameters used are given in Methods. CCA blood flow levels are shown with intact CST (Control; open column, n = 7) and during repetitive stimulation of the CST (after its section) at various frequencies (0.5-10 Hz; filled columns, n = 7). The hatched portions of the columns indicate the increase in CCA blood flow evoked by LN stimulation during repetitive CST stimulation at various frequencies (0-10 Hz). The CCA blood flow levels are expressed on the ordinate in absolute terms (ml min−1 kg−1). Values shown are means ± s.e.m.*P < 0.01, **P < 0.001 vs. before section of the CST (Control; ANOVA followed by a contrast test); *P < 0.05, **P < 0.01 vs. 0 Hz CST stimulation (ANOVA followed by a contrast test). †P < 0.001, significant difference between the increase in blood flow recorded on LN stimulation during 0 Hz CST stimulation and that during repetitive CST stimulation (at 10 Hz) (ANOVA for repeated measurements followed by a contrast test). The bracket (P < 0.001) indicates a significant difference between the two columns (Student's paired t test).
Figure 4. Mean data (±s.e.m.) for effects of electrical stimulation.
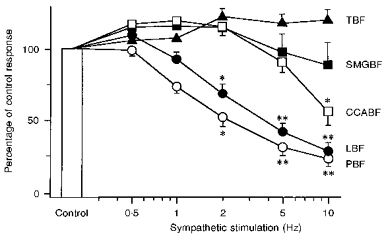
Effects of electrical stimulation of the central cut end of the LN on blood flow in lower lip (LBF, •, n = 11), palate (PBF, ^, n = 5), SMG (SMGBF, ▪, n = 4) and CCA (CCABF, □, n = 8), and of the peripheral cut end of the LN on tongue blood flow (TBF, ▴, n = 7). The above nerves were stimulated alone (Control) or during on-going repetitive CST stimulation in vago-sympathectomized cats. Experimental conditions were as in Fig. 3. The LN-stimulated blood flow increases during CST stimulation are expressed as a percentage of the response to LN stimulation in the absence of CST stimulation (Control). Statistical significance was calculated by means of ANOVA followed by a contrast test. *P < 0.01, **P < 0.001 vs. the response in the absence of CST stimulation (Control).
Measurement of lower lip, palate, SMG and tongue blood flows, and of systemic arterial blood pressure
Changes in blood flow at sites in the palate, in the lower lip adjacent to the canine tooth, and in the SMG and tongue were monitored on either side using a laser-Doppler flowmeter (LDF; Advance ALF21R, Tokyo, Japan) as described before (Izumi & Karita, 1990, 1993, 1994, 1995). The probe was placed against the lower lip, palate or tongue without exerting any pressure on the tissues. The LDF values obtained in this way represent the blood flow in the superficial vessels in each tissue. Electrical calibration for zero blood flow was performed for all recordings. Several gains were selectable and the maximum output of a given gain level (defined electrically) was taken as 100%. The analog output of the equipment does not give absolute values, but shows relative changes in blood flow (for technical details and evaluation of the LDF method see Stern et al. 1977). Blood flow was recorded from the common carotid artery (CCA) by means of an electromagnetic blood flow transducer fitted to an electromagnetic blood flowmeter (EMF; model AT-610G, Nihon Koden) as described before (Izumi et al. 1997). Output from the devices was continuously displayed on an eight-channel chart recorder (Graphtec, model W5000, Tokyo, Japan) at a speed of 10 mm min−1. The blood flow changes were assessed by measuring the height of the response.
Systemic arterial blood pressure was recorded from the femoral catheter via a Statham pressure transducer. A tachograph (Nihon Koden model AT-610G) triggered by the arterial pulse was used to monitor heart rate.
Statistical analysis
All numerical data are given as means ±s.e.m. The significance of changes in the responses was assessed using either Student's paired t test or analysis of variance (ANOVA) followed by a contrast test. Differences were considered significant at P < 0.05. Data were analysed using a Macintosh Computer with StatView 4.5 and Super ANOVA (Abacus Concepts, CA, USA).
RESULTS
Effect of section and stimulation of the CST on CCA blood flow
Figure 2 shows the effect of section of the CST on the basal CCA blood flow. Section of the ipsilateral CST increased the basal CCA blood flow significantly (from 12.1 ± 1.4 to 16.1 ± 1.3 ml min−1 kg−1, n = 7; P < 0.001, Student's paired t test). Electrical stimulation of the peripheral cut end of the CST at frequencies of 0.5-10 Hz decreased the basal CCA blood flow in a frequency-dependent manner (F(6,36) = 50.68; P < 0.001, ANOVA test for repeated measurements). The level of the basal CCA blood flow under control conditions was comparable with the levels observed when repetitive CST stimulation was performed at 0.5-1 Hz, suggesting that in the CST fibres that innervate the vascular beds supplied by the CCA, the spontaneous discharge was around 0.5-1 Hz.
Interaction between the effect of LN stimulation on CCA blood flow and that of CST stimulation
Electrical stimulation of the central cut end of the LN (always at 10 Hz) elicited an increase in CCA blood flow during CST stimulation at 0-10 Hz (the full range of frequencies used). No statistically significant difference was found between the increase in CCA blood flow evoked by LN stimulation alone and that evoked by LN stimulation during CST stimulation, provided the frequency of CST stimulation was 5 Hz or less (compare the hatched areas in Fig. 2). By contrast, the LN-evoked increase in CCA blood flow was markedly reduced by CST stimulation at a frequency of 10 Hz (P < 0.001; ANOVA followed by a contrast test).
Effects on tissue blood flow of CST section and LN stimulation during on-going CST stimulation
Section of the CST caused little or no change in basal blood flow (measured using the LDF method) in the ipsilateral lower lip, palate, SMG or tongue, the blood flow levels in these tissues being 98.7 ± 1.4, 102.1 ± 1.7, 98.3 ± 1.2 and 100.4 ± 1.2 %, respectively (n = 5 in each case) of the levels recorded before CST section. Typical examples of individual blood flow responses in the lower lip, palate, SMG and CCA evoked during on-going CST stimulation at frequencies of 0.5-10 Hz when the LN was stimulated centrally (or peripherally in the case of tongue blood flow) are shown in Fig. 3A and B. Mean data are shown in Fig. 4. Figure 3C shows typical examples of the effect on tongue blood flow when the central cut end of the inferior alveolar nerve was electrically stimulated during on-going CST stimulation. The blood flow responses at all the sites examined were highly reproducible over the period of study; in fact, this reliability persisted for more than 6 h. Although the following was not the case in the experiment shown in Fig. 3, the different frequencies of CST stimulation (0.5-10 Hz) were normally applied at random, because of the possibility that the response to a given LN stimulation might be affected by the previous period of CST stimulation. However, in the present study, the order in which the different frequencies were presented had no statistically significant effect on the magnitude of the LN-evoked responses. This was true for all the measurement sites examined.
Figure 3. Individual examples of the effects of electrical stimulation.
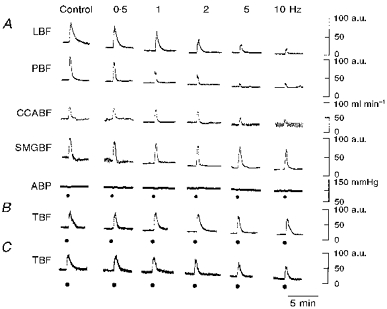
A, effects of electrical stimulation of the central cut end of the LN on blood flow in lower lip (LBF), palate (PBF), CCA (CCABF) and SMG (SMGBF). B and C, effects of electrical stimulation of the peripheral cut end of the LN (B), and of the central cut end of the inferior alveolar nerve (C) on tongue blood flow (TBF). The above stimuli were delivered alone (Control) or during on-going repetitive CST stimulation in vago-sympathectomized cats. The LN was stimulated where indicated (•) for 20 s at a supramaximal voltage (30 V) at 10 Hz with pulses of 2 ms duration. The frequencies (0.5-10 Hz) of CST stimulation are shown at the top. ABP, systemic arterial blood pressure. a.u., arbitrary units.
The basal blood flow at all the sites examined was reduced by CST stimulation in a frequency-dependent manner. In the lower lip and palate, the LN-evoked blood flow increases were markedly reduced by concurrent CST stimulation in a frequency-dependent manner (for lower lip, F(5,36) = 41.68, n = 11, P < 0.001; for palate, F(5,20) = 27.40, n = 5, P < 0.001; both by ANOVA for repeated measurements). However, this was not the case for the tongue (on stimulation of the peripheral cut end of LN) or SMG (on stimulation of the central cut end of LN) (for tongue, F(5,30) = 4.68, n = 7, not significant; for SMG, F(5,15) = 2.38, n = 4, not significant). The basal blood flow level in the tongue was decreased by electrical stimulation of the CST in a frequency-dependent manner regardless of whether the LN was (Fig. 3B) or was not (Fig. 3C) cut, indicating that sympathetic vasoconstrictor fibres from the CST to the tongue run separately from the LN.
A statistically significant reduction in the CCA blood flow response to LN stimulation was only seen on CST stimulation at 10 Hz (P < 0.01, ANOVA followed by a contrast test), indicating that the LN-evoked changes in CCA blood flow behaved in a fashion different from those seen in the lower lip, palate, tongue and SMG. Like the effects of direct parasympathetic stimulation on the tongue (Figs 3B and 4), the tongue blood flow increase elicited reflexly by stimulation of the central cut end of the inferior alveolar nerve was not inhibited by CST stimulation at any frequency used (Fig. 3C). These results seem to indicate a lack of ability of CST stimulation to attenuate evoked blood flow increases in the tongue, regardless of the method used to evoke the increases.
The changes in arterial blood pressure following either CST stimulation (at any frequency examined) or LN stimulation were too slight to account for the blood flow changes measured by the present methods (0.4 ± 0.4, 2.5 ± 1.3, 2.7 ± 1.9, 3.2 ± 2.1 and 4.8 ± 2.3 mmHg, respectively, for CST stimulation at 0.5, 1, 2, 5 and 10 Hz (n = 10) or -8.8 ± 2.9 mmHg (n = 8) for LN stimulation). Indeed, the blood pressure changes showed no relation to the blood flow changes (for examples, see Fig. 3).
DISCUSSION
Blood flow in lower lip, palate, SMG, tongue, gingiva and CCA is considered to be regulated by both sympathetic and parasympathetic efferent nerves (Izumi et al. 1990; Izumi & Karita, 1990, 1992, 1993, 1994, 1995; Izumi, 1995; Karita & Izumi, 1995). In fact, electrical stimulation of sympathetic efferents elicits vasoconstriction, whereas stimulation of parasympathetic efferents elicits vasodilatation in all the oro-facial tissues so far examined. However, we found in our preliminary experiments in cats that no sympathetically mediated vasoconstrictor responses occurred reflexly in oro-facial tissues in response to a variety of stimuli (such as trigeminal, gustatory and visceral stimulation) in marked contrast to the parasympathetically mediated reflex vasodilatation evoked by the same stimuli. In addition, we already knew that while repetitive stimulation of the CST in the cat causes a frequency-dependent augmentation of parasympathetic reflex salivary secretion, it does not affect the accompanying blood flow response in the SMG (Izumi & Karita, 1995). These results raised a question about the physiological role of the sympathetic nervous system in the control of blood vessels in this area.
As shown in Figs 3 and 4, electrical stimulation of the preganglionic fibres to the superior cervical sympathetic ganglion in the cat caused a frequency-dependent attenuation of the parasympathetically mediated (LN-evoked) blood flow increase in the lower lip, palate and CCA, but not in the tongue or SMG. The attenuating effect of high frequencies of CST stimulation on the parasympathetically mediated blood flow increases in lower lip and palate is somewhat similar to the attenuating effect of sympathetic vasoconstriction on antidromic vasodilatation. The latter effect of sympathetic activation was recently described by Habler et al. (1997) who evoked antidromic vasodilatation in the rat skin by electrical stimulation of the appropriate dorsal root. The results reported here suggest that whether such an attenuation is induced by CST stimulation in the oro-facial area depends on the tissue under study.
Following section of the left CST, the ipsilateral CCA blood flow (measured by EMF) was consistently enhanced. On average, the flow increased by 3.96 ± 0.30 ml min−1 kg−1 (Fig. 2), corresponding to a 36.2 ± 5.8 % increase (n = 7, P < 0.001, Student's paired t test). This is in harmony with the observation of Lacroix et al. (1994b) that internal maxillary arterial blood flow (recorded using an ultrasonic flowmeter) was enhanced by nearly 50 % following section of the CST. However, in the present study the blood flow (measured by LDF) in lower lip, palate, SMG and tongue was largely unaffected by section of the CST. This might seem to suggest that the sympathetic vasoconstrictor fibres supplying these tissues have either no or a very low level of activity under the present experimental conditions. However, tonically active vasoconstrictor neurones have been reported to be present in the superior cervical ganglion of the rat (Bartsh et al. 1996; McLachlan et al. 1997). The precise reasons for these differences are unclear, although it should be pointed out (i) that the above flow measurements were made using three fundamentally different methods and that these methods measure different sections of the vascular bed, and (ii) that the LDF method is not well suited to the measurement of absolute blood flow levels.
As mentioned in Results, our data (Fig. 2) seem to indicate that, in the fibres of the CST that innervate the vascular beds supplied by the CCA, the spontaneous discharge is around 0.5-1 Hz under our experimental conditions. This conclusion is consistent with (i) the observation of Habler et al. (1994) that the frequency of extracellularly recorded spontaneously active postganglionic units in the rat hindlimb is ∼1 Hz, and (ii) the finding of Bartsch et al. (1996) that active superior cervical ganglion neurones projecting to the salivary glands (probably constituting the vasoconstrictor population) discharged at frequencies with a mean of 0.7 Hz.
The precise mechanism by which sympathetic stimulation attenuates the parasympathetic vasodilatation in the lower lip and palate, but not that in the tongue or SMG, is unclear. Any one of several different types of interaction could be involved. Since we have no data in favour of any particular one, speculation about the nature of the mechanism would be unwarranted at present.
It was recently reported by Lacroix et al. (1994b) that stimulation of the CST at 10 Hz for 3 min in the cat causes a long-lasting (more than 30 min) attenuation of subsequent parasympathetically mediated nasal vasodilatation (evoked by electrical stimulation of the vidian nerve). However, in the present study we could find no evidence of such a long-lasting inhibitory effect. Indeed, we found that LN-evoked reflex responses recovered rapidly (within 10 min) after the cessation of CST stimulation at 10 Hz for 10 min (data not shown).
It is to be hoped that further studies on the precise mechanism underlying the sympathetic attenuation of parasympathetic vasodilatation will provide data enabling a better understanding of the interaction between the effects of sympathetic and parasympathetic nerves and its importance in blood flow regulation in oro-facial areas.
Acknowledgments
This study was supported by a Grant-in-Aid for scientific research from the Ministry of Education, Science, Sports and Culture of Japan (no. 08672115, no. 09557161 and no. 09671886; to H. Izumi).
References
- Bartsch T, Habler H-J, Jänig W. Functional properties of postganglionic sympathetic neurones supplying the submandibular gland in the anaesthetized rat. Neuroscience Letters. 1996;214:143–146. doi: 10.1016/0304-3940(96)12910-4. [DOI] [PubMed] [Google Scholar]
- Gardner TD, Potter EK. Dependence of non-adrenergic inhibition of cardiac vagal action on peak frequency of sympathetic stimulation in the dog. The Journal of Physiology. 1988;405:115–122. doi: 10.1113/jphysiol.1988.sp017324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habler H-J, Jänig W, Krummel M, Peters OA. Reflex patterns in postganglionic neurons supplying skin and skeletal muscle of the rat hindlimb. Journal of Neurophysiology. 1994;72:2222–2236. doi: 10.1152/jn.1994.72.5.2222. [DOI] [PubMed] [Google Scholar]
- Habler H-J, Wasner G, Jänig W. Interaction of sympathetic vasoconstriction and antidromic vasodilatation in the control of skin blood flow. Experimental Brain Research. 1997;113:402–410. doi: 10.1007/pl00005594. [DOI] [PubMed] [Google Scholar]
- Hall GT, Gardner TD, Potter EK. Attenuation of long-lasting effects of sympathetic stimulation after repeated stimulation. Circulation Research. 1990;67:193–198. doi: 10.1161/01.res.67.1.193. [DOI] [PubMed] [Google Scholar]
- Izumi H. Reflex parasympathetic vasodilatation in facial skin. General Pharmacology. 1995;26:237–244. doi: 10.1016/0306-3623(94)00155-g. 10.1016/0306-3623(94)00155-G. [DOI] [PubMed] [Google Scholar]
- Izumi H, Karita K. The effects of capsaicin applied topically to inferior alveolar nerve on antidromic vasodilatation in cat gingiva. Neuroscience Letters. 1990;112:65–69. doi: 10.1016/0304-3940(90)90323-2. 10.1016/0304-3940(90)90323-2. [DOI] [PubMed] [Google Scholar]
- Izumi H, Karita K. Somatosensory stimulation causes autonomic vasodilatation in cat lip. The Journal of Physiology. 1992;450:191–202. doi: 10.1113/jphysiol.1992.sp019123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi H, Karita K. Innervation of the cat lip by two groups of parasympathetic vasodilator fibres. The Journal of Physiology. 1993;465:501–512. doi: 10.1113/jphysiol.1993.sp019690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi H, Karita K. The parasympathetic vasodilator fibers in the trigeminal portion of the distal lingual nerve in the cat tongue. American Journal of Physiology. 1994;266:R1517–1522. doi: 10.1152/ajpregu.1994.266.5.R1517. [DOI] [PubMed] [Google Scholar]
- Izumi H, Karita K. Low-frequency subthreshold sympathetic stimulation augments maximal reflex parasympathetic salivary secretion in cats. American Journal of Physiology. 1995;268:R1188–1195. doi: 10.1152/ajpregu.1995.268.5.R1188. [DOI] [PubMed] [Google Scholar]
- Izumi H, Kuriwada S, Karita K, Sasano T, Sanjo D. The nervous control of gingival blood flow in cats. Microvascular Research. 1990;39:94–104. doi: 10.1016/0026-2862(90)90061-u. 10.1016/0026-2862(90)90061-U. [DOI] [PubMed] [Google Scholar]
- Izumi H, Sato M, Karita K, Iwatsuki N. Blood flow increases in common carotid artery, lower lip and palate elicited by lingual nerve stimulation in anesthetized cats. Journal of the Autonomic Nervous System. 1997;62:167–173. doi: 10.1016/s0165-1838(96)00123-3. 10.1016/S0165-1838(96)00123-3. [DOI] [PubMed] [Google Scholar]
- Karita K, Izumi H. Effect of baseline vascular tone on vasomotor responses in cat lip. The Journal of Physiology. 1995;482:679–685. doi: 10.1113/jphysiol.1995.sp020550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerezoudis NP, Funato A, Edwall L, Olgart L. Activation of sympathetic nerves exerts an inhibitory influence on afferent nerve-induced vasodilation unrelated to vasoconstriction in rat dental pulp. Acta Physiologica Scandinavica. 1993;147:27–35. doi: 10.1111/j.1748-1716.1993.tb09469.x. [DOI] [PubMed] [Google Scholar]
- Lacroix JS, Ulman LG, Potter EK. Modulation by neuropeptide Y of parasympathetic nerve-evoked nasal vasodilatation via Y2 prejunctional receptor. British Journal of Pharmacology. 1994a;113:479–484. doi: 10.1111/j.1476-5381.1994.tb17014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix JS, Ulman LG, Potter EK. Sympathetic and parasympathetic interaction in vascular control of the nasal mucosa in anaesthetized cats. The Journal of Physiology. 1994b;480:325–331. doi: 10.1113/jphysiol.1994.sp020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan EM, Davies PJ, Häbler H-J, Jamieson J. On-going and reflex synaptic events in rat superior cervical ganglion cells. The Journal of Physiology. 1997;501:165–182. doi: 10.1111/j.1469-7793.1997.165bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty M, Gibbins IL, Potter EK, McCloskey DI. Comparison of the inhibitory roles of neuropeptide Y and galanin on cardiac vagal action in the dog. Neuroscience Letters. 1992;139:275–279. doi: 10.1016/0304-3940(92)90570-w. 10.1016/0304-3940(92)90570-W. [DOI] [PubMed] [Google Scholar]
- Olgart L, Kerezoudis NP. Nerve-pulp interactions. Archives of Oral Biology. 1994;39:47S–54S. doi: 10.1016/0003-9969(94)90188-0. 10.1016/0003-9969(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Potter EK. Prolonged non-adrenergic inhibition of cardiac vagal action following sympathetic stimulation: neuromodulation by neuropeptide Y? Neuroscience Letters. 1985;54:117–121. doi: 10.1016/s0304-3940(85)80065-3. [DOI] [PubMed] [Google Scholar]
- Revington M, Potter EK, McCloskey DI. Prolonged inhibition of cardiac vagal action following sympathetic stimulation and galanin in anaesthetized cats. The Journal of Physiology. 1990;431:495–503. doi: 10.1113/jphysiol.1990.sp018342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern MD, Lappe DL, Bowen PD, Chimosky JE, Holloway GAJ, Keiser HR, Bowman RL. Continuous measurement of tissue blood flow by laser-Doppler spectroscopy. American Journal of Physiology. 1977;232:H441–448. doi: 10.1152/ajpheart.1977.232.4.H441. [DOI] [PubMed] [Google Scholar]
- Ulman LG, Moriarty M, Potter EK, McCloskey DI. Galanin antagonist effects on cardiac vagal inhibitory actions of sympathetic stimulation in anaesthetized cats and dogs. The Journal of Physiology. 1993;464:491–499. doi: 10.1113/jphysiol.1993.sp019647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulman LG, Potter EK, McCloskey DI. Effects of sympathetic activity and galanin on cardiac vagal action in anaesthetized cats. The Journal of Physiology. 1992;448:225–235. doi: 10.1113/jphysiol.1992.sp019038. [DOI] [PMC free article] [PubMed] [Google Scholar]


