Abstract
Transcranial magnetic stimulation (TMS) of the human motor cortex results in multiple discharges (D and I waves) in the corticospinal tract. We tested whether these volleys can be explored non-invasively with paired TMS. The intensity of the first stimulus (S1) was set to produce a motor-evoked potential (MEP) of 1 mV in the resting contralateral abductor digiti minimi (ADM) muscle; the second stimulus (S2) was set to 90 % of the resting motor threshold. At interstimulus intervals of 1·1-1·5, 2·3-2·9 and 4·1-4·4 ms the MEP elicited by S1 plus S2 was larger than that produced by S1 alone.
Varying the S1 intensity between 70 and 130 % resting motor threshold with S2 held constant at 90 % resting motor threshold showed that the threshold for the first MEP peak was <= 70 % resting motor threshold. The second and third MEP peaks appeared only at higher S1 intensities. The latency of all peaks decreased with increasing S1 intensity.
Varying the S2 intensity with S1 held constant to produce a MEP of 1 mV on its own showed that the amplitude of all MEP peaks increased with S2 intensity, but that their timing remained unchanged.
Paired TMS in the active ADM (S1 clearly suprathreshold, S2 just above threshold; interstimulus interval, 1 ms) produced strong MEP facilitation. The onset of this facilitation occurred later by about 1·5 ms than the onset of the MEP evoked by S2 alone. No MEP facilitation was seen if the magnetic S2 was replaced by anodal or cathodal transcranial electrical stimulation.
It is concluded that the MEP facilitation after paired TMS, at least for the first MEP peak, is due to facilitatory interaction between I waves, and takes place in the motor cortex at or upstream from the corticospinal neurone.
Patton & Amassian (1954) were the first to describe multiple descending volleys in the corticospinal tract after motor cortex stimulation in cats and monkeys. They provided evidence that the first wave was produced by direct excitation of corticospinal neurones while later waves originated from indirect activation via cortical interneurones. According to this hypothesis, the terms D (direct) and I (indirect) wave were coined. In humans, the D and I waves produced by transcranial electrical stimulation (TES) or transcranial magnetic stimulation (TMS) have been recorded intraoperatively from the spinal cord using electrodes inserted into the epidural space (e.g. Boyd et al. 1986; Hicks et al. 1992; Burke et al. 1993; Rothwell et al. 1994; Fujiki et al. 1996). Single motor unit recordings provide a less invasive technique for the study of D and I waves (e.g. Day et al. 1989; Boniface et al. 1991; Mills, 1991), and F waves (Mercuri et al. 1996) and H reflexes (Cowan et al. 1986; van der Linden & Bruggeman, 1993; Mazzocchio et al. 1994) conditioned by TMS or TES provide a completely non-invasive means. However, all the latter techniques look primarily at the excitability of spinal motoneurones and are unable to distinguish between the contributions of direct and indirect activation of the corticospinal tract to the changes in excitability observed at the spinal segment.
In the present study, we propose a paired TMS protocol as a novel non-invasive approach for the investigation of I waves. The protocol is essentially different from previously reported paired-TMS protocols (Kujirai et al. 1993; Ziemann et al. 1996) since it employs a suprathreshold first, and a subthreshold second stimulus, rather than a subthreshold conditioning and a suprathreshold test stimulus. Only one recent paper has described a somewhat similar technique using two near-threshold stimuli (Tokimura et al. 1996). These authors concluded that facilitation between the two magnetic stimuli at short interstimulus intervals took place mainly at the cortical level, but they did not offer a detailed explanation for the mechanisms responsible for this facilitation.
METHODS
Nine healthy volunteers (mean age, 29.8 ± 5.6 years; range, 20-41 years; 3 women, 6 men) participated in the experiments. Written informed consent was obtained from all subjects. Studies were performed according to the Declaration of Helsinki and all procedures were approved by the ethics committees of the Medical Faculty of the University of Göttingen and the National Institute of Neurological Disorders and Stroke.
Subjects were seated in a comfortable reclining chair. Surface electromyogram (EMG) recordings were made from the right abductor digiti minimi (ADM) muscle using Ag-AgCl cup electrodes. The active electrode was placed over the motor point and the reference electrode 4-5 cm distally on the little finger. The raw EMG signal was amplified and filtered with a time constant of 10 ms and a low-pass filter of 2.5 kHz. Signals were then digitized (sampling rate, 5 kHz) and fed into an IBM-PC/486 AT-compatible laboratory computer, using the NEUROSCAN (version 3.0; Neuroscan Inc., Sterling, VA, USA) data collection and conditional averaging software.
TMS was performed using two Magstim 200 HP (Magstim, Whitland, Dyfed, UK) magnetic stimulators which were connected through a BiStim module to one figure-of-eight magnetic coil (diameter of each loop, 70 mm; peak magnetic field, ∼1.5 T). The coil was placed flat on the head with the handle pointing backwards and rotated about 45 deg away from the mid-line. Thus, the current induced in the brain was directed approximately perpendicular to the central sulcus. This orientation of the induced electrical field is thought to be optimal for a predominantly trans-synaptic mode of activation of the corticospinal system (Kaneko et al. 1996; Nakamura et al. 1996). The optimal coil position for activation of the target muscle was determined as the site where stimulation consistently produced the largest motor-evoked potentials (MEPs) at a slightly suprathreshold stimulus intensity. This site was marked with a pen in order to ensure consistent placement of the coil throughout the experiment. Resting motor threshold was determined to the nearest 1 % of maximum stimulator output using single TMS pulses, but with the stimulator connected to the coil through the BiStim module. Resting motor threshold was defined as the minimum stimulus intensity which elicited at least five out of ten MEPs > 50 μV at a high recording gain. Active motor threshold was measured in the ADM during an isometric contraction. Five consecutive trials were averaged in order to distinguish small MEPs from the on-going voluntary EMG activity. Active motor threshold was defined as the minimum intensity required to produce an averaged MEP > 50 μV. The force level was 20 % of the maximum voluntary contraction. Isolation of the ADM and measurement of the force were achieved by positioning the right forearm in pronation on an arm rest, fixing the forearm at the elbow and wrist with straps, and attaching a strain gauge to the little finger. The force level was fed back to the subject via an oscilloscope and the EMG was played through a loudspeaker. Breaks were allowed in order to avoid fatigue.
Most paired-TMS experiments were done in the resting ADM. For the main experiment, the intensity of the first stimulus (S1) was set to produce a MEP with a mean peak-to-peak amplitude of 1 mV when given alone, corresponding to an activation of approximately 5-10 % of the total spinal motoneurone pool. The intensity of the second stimulus (S2) was set to 90 % of the resting motor threshold. Interstimulus intervals from 0.5 to 5.2 ms were tested in 0.1 ms steps (48 intervals). Each session consisted of twelve blocks of forty trials each. Each block was composed of five conditions presented eight times each: control (S1 given alone) and four paired conditions (S1 plus S2) at different interstimulus intervals. The order of conditions within blocks and the order of blocks within sessions were pseudorandomized. Means of the peak-to-peak MEP sizes were calculated for each condition, and the size of the MEP elicited by S1 plus S2 was then expressed as a percentage of the mean size of the control MEPs obtained in the same block. This experiment was conducted in five subjects. Each subject was tested three times on different days, in order to evaluate intraindividual reproducibility. For all experiments, the time between consecutive trials was 5 s.
The effects of varying the intensity of S1 and S2 were studied in similar experiments. The tested range of interstimulus intervals was 0.5-5.1 ms; the intervals were varied in 0.2 ms steps (24 intervals, studied in 6 blocks containing 1 control and 4 paired conditions each). In one set of experiments (6 subjects), S1 was varied while S2 was kept constant at 90 % resting motor threshold. The intensities of S1 tested were 70, 90, 100, 110 and 130 % of resting motor threshold. In another set of experiments, which was carried out in three subjects, the intensity of S1 was set to produce a control MEP of about 1 mV, while S2 was varied as follows: 90 % of active motor threshold, 90 % of resting motor threshold, 120 % of resting motor threshold, equal to S1 and 120 % of S1. Only one stimulus intensity was tested per session. The order of stimulus intensities across sessions was pseudorandomized.
In order to study in more detail the mechanisms responsible for the first MEP peak that was observed in the above experiments, paired TMS or a combination of TMS (S1) and TES (S2) were given in two subjects at an interstimulus interval of 1 ms while the ADM was voluntarily activated at 20 % of maximum voluntary contraction. The intensity of S1 was set so as to produce a mean MEP of about 1 mV in peak-to-peak amplitude, while S2 was slightly above active motor threshold. For TES, a Digitimer D180 electrical stimulator was used with a time constant of 100 μs. For anodal TES, the anode was placed 6-7 cm lateral to the mid-line and 1-2 cm anterior to the vertex, while the cathode was fixed at the vertex. For cathodal TES, the electrodes were reversed. Ten trials were performed per condition in a pseudorandomized order. The MEPs to paired stimulation were superimposed on the MEPs elicited by S1 and S2 alone in order to determine the onset of MEP facilitation. These experiments were done in the active ADM in order to eliminate the spinal motoneurones as a potentially confounding site of facilitatory interaction between the effects of S1 and S2 at the onset of facilitation.
The effect of voluntary contraction of the ADM (20 % maximum voluntary contraction) on the full range of interstimulus intervals (0.5-5.1 ms in 0.2 ms steps) was explored in another set of experiments run on the same five subjects as in the main experiment with the ADM at rest, and tested three times each. S1 was adjusted to produce a control MEP of about 1 mV when given alone, while the intensity of S2 was set at 90 % of active motor threshold.
Since in most experiments two magnetic stimuli were delivered through the same coil at very short interstimulus intervals, we sought to exclude technical factors, namely an alteration of the amplitude of S2 by S1, as a possible cause of the observed increase in MEP size. We recorded the voltage induced in a search coil by the two stimuli. The induced voltage from S2 was slightly attenuated at interstimulus intervals of 0.5-0.9 ms. However, similar to the findings of (1996 Tokimura et al., see Fig. 8), we found that at intervals of 0.5-1.2 ms the current induced by S2 showed a very brief transient above the peak produced by S1. At intervals > 1.2 ms, there were no obvious alterations in current size. The physiological significance of the inequalities between S1 and S2 at the very short intervals is not clear. Therefore, we compared in two subjects the effects of paired-pulse stimulation on MEP size between two orthogonal coil orientations with the current induced in the brain directed either postero-anterior or latero-medial. The latter predominantly activates the corticospinal system directly (Kaneko et al. 1996). In both subjects, the postero-anteriorly directed current produced a marked MEP facilitation that was lacking with the medio-lateral current (data not shown). Therefore, it is unlikely that the very brief transient current produced by S2 at intervals < 1.2 ms contributed significantly to the findings of the present paper.
Statistical procedures
In most experiments, the size of the MEP evoked by S1 plus S2 was expressed as a percentage of the control MEP elicited by S1 alone. In order to evaluate at which interstimulus intervals these percentages were different from 100 %, multiple Student's one-sample two-tailed t tests (hypothesized mean = 100) were performed for all intervals tested, and a Bonferroni correction for multiple comparisons was made. For the experiments where the intensity of S1 was varied, it was necessary to use the absolute size of the MEP, since some S1 intensities were subthreshold and, therefore, did not produce a control MEP on their own. The MEP sizes were individually normalized to the maximum MEP across all interstimulus intervals tested, which was assigned a value of 1. Then, multiple, Bonferroni-corrected, one-sample one-tailed t tests were calculated and those intervals not significantly different from 1 were considered as peaks. For all experiments, statistical significance was assumed if P < 0.05.
RESULTS
The principal findings are illustrated in Fig. 1A-C. A magnetic stimulus (S1) was adjusted to produce a MEP of about 1 mV in the voluntarily relaxed ADM. This MEP was facilitated if a weak second magnetic stimulus (S2) that did not produce a MEP when given alone followed S1 at interstimulus intervals of 1.1-1.5, 2.3-2.9 and 4.1-4.4 ms (Fig. 1A and B). These three MEP peaks were separated by troughs where S2 had no significant effect on MEP size. Figure 1C shows that this succession of peaks and troughs was reproducible within individuals. Furthermore, the timing of the peak centres (the central interval out of five neighbouring interstimulus intervals yielding the maximum sum of MEP sizes in a given peak) showed a low variability across subjects. The centre of the first peak was observed at intervals between 1.2 and 1.3 ms, the second peak at 2.5-2.8 ms and the third peak at 4.2-4.6 ms. The interpeak intervals varied from 1.2 to 1.6 ms between the first and second MEP peaks, and from 1.4 to 2.1 ms between the second and third peaks (Fig. 1C).
Figure 1. Motor-evoked potential facilitation by paired transcranial magnetic stimulation in the relaxed abductor digiti minimi muscle as a function of the interstimulus interval.
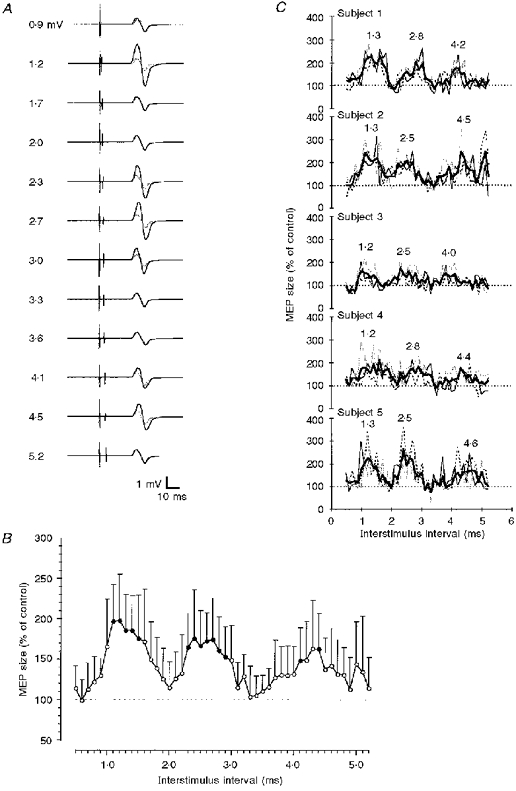
A, MEPs in a single subject. Motor responses to the first magnetic stimulus alone (control MEP) are shown in grey; motor responses to the same stimulus followed by a second stimulus (set to 90 % resting motor threshold) are shown in black. Interstimulus intervals increase from top to bottom, as indicated on the left. All recordings are averages from 8 single trials. Note that the motor response was facilitated at interstimulus intervals of 1.2, 2.3-3.0 and 4.1-4.5 ms. B, averaged data from 5 subjects tested 3 times each. The abscissa indicates the interstimulus interval (0.1 ms steps). MEP size after paired stimulation is expressed as a percentage of the control MEP on the ordinate. The dotted horizontal line indicates the 100 % level (no effect by the second stimulus). Error bars are standard deviations. • denotes a statistically significant facilitation (P < 0.05; Bonferroni-corrected multiple t tests). C, individual time course data for the 3 sessions in B. The thin black, grey and dashed lines refer to the first, second and third sessions, respectively. The mean across sessions for a given subject is shown by the thick black lines. Numbers above the curves denote the interstimulus intervals at the centres of the MEP peaks.
The timing and magnitude of the MEP peaks as a function of the intensity of S1 are shown in Fig. 2. These experiments were carried out with S2 set to 90 % of resting motor threshold. At the lowest intensity of S1 tested (70 % of resting motor threshold), one small MEP peak occurred at interstimulus intervals of 1.7-1.9 ms. If the intensity of S1 was increased to 90 % of resting motor threshold, this first peak increased in amplitude and advanced to interstimuls intervals of 1.3-1.5 ms. Furthermore, a second peak occurred at an interstimulus interval of 3.5 ms. With the intensity of S1 equal to resting motor threshold, the second peak advanced to an interstimulus interval of 3.1 ms. When the intensity of S1 was further increased to 110 % of resting motor threshold, both the first and second peaks occurred at even shorter intervals, of 1.1-1.5 ms and 2.7 ms, respectively, and a late third peak appeared at an interval of 4.9 ms. Finally, at the highest intensity of S1 tested (130 % of resting motor threshold, which was about equal to the intensity used in the experiments in Fig. 1), three MEP peaks were clearly distinguishable at interstimulus intervals of 1.3-1.9, 2.3-2.9 and 4.3 ms, respectively.
Figure 2. Motor-evoked potential facilitation by paired transcranial magnetic stimulation as a function of the intensity of the first stimulus.
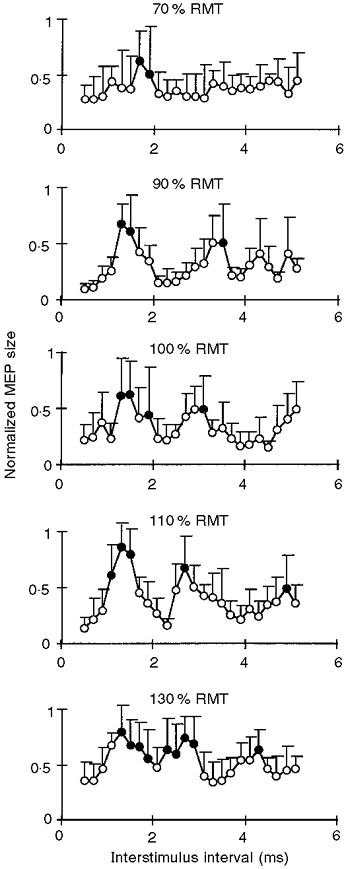
Averaged motor responses in the relaxed ADM of 6 subjects are plotted against the interstimulus interval at different intensities of the first stimulus (indicated at the top of each panel; RMT, resting motor threshold). Error bars indicate standard deviation. The intensity of the second stimulus was fixed at 90 % of RMT. MEP size was normalized for a given subject and a given session to the maximum MEP, which was assigned a value of 1. • denotes MEPs which were not significantly different from 1 (P > 0.05, Bonferroni-corrected multiple t tests).
The timing and amplitude of the MEP peaks as a function of the intensity of S2 are shown in Fig. 3. S1 was kept at an intensity that produced a MEP of about 1 mV when given alone. At least the first two peaks were already clearly visible at the lowest intensity of S2 tested (90 % of active motor threshold). The amplitudes of the MEP peaks increased with S2 intensity. In contrast, the timing of the peaks and troughs was not altered by changes in the S2 intensity (Fig. 3B), and was comparable to the findings in the experiments in Fig. 1. At intensities of S2 equal to (⋄, Fig. 3A) or above S1 (□, Fig. 3A), S2 also had some facilitatory effect on MEP size during the troughs.
Figure 3. Motor-evoked potential facilitation by paired transcranial magnetic stimulation as a function of the intensity of the second stimulus.
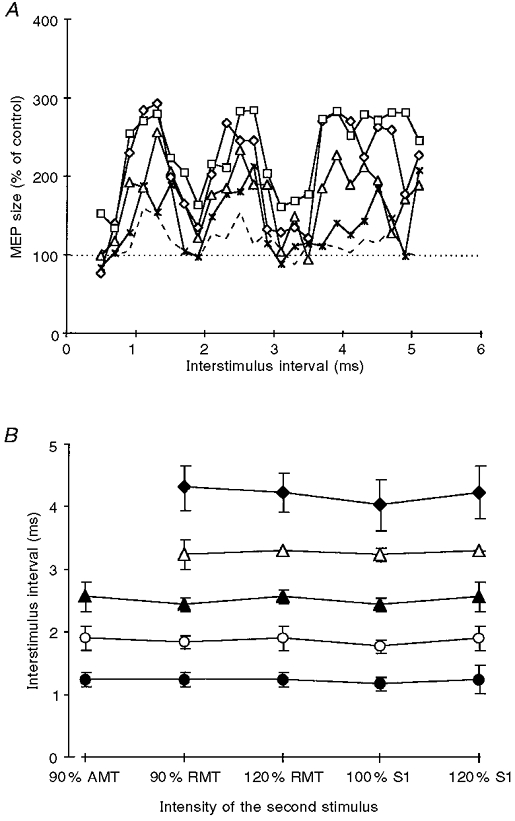
A, averaged motor responses in the relaxed ADM of 3 subjects are plotted against the interstimulus interval. MEP size after paired stimulation is expressed as a percentage of the control MEP to the first stimulus alone. The intensity of the first stimulus was fixed to produce a MEP of 1 mV; the intensity of the second stimulus was 90 % of active motor threshold (AMT, dashed line), 90 % of resting motor threshold (RMT, ×), 120 % of resting motor threshold (▵), the same intensity as the first stimulus (⋄), or 120 % of the first stimulus (□). The horizontal dotted line indicates the 100 % level (i.e. no effect by the second stimulus). B, averaged (3 subjects) interstimulus intervals where the centre of the 3 MEP peaks (P1, P2 and P3, denoted as •, ▴ and ♦, respectively) and the centre of the two troughs (T1 and T2, denoted as ○ and ▵, respectively) occurred. Centres were defined as the central interval out of those 3 neighbouring interstimulus intervals providing maximum (for peaks) or minimum (for troughs) sums of MEP values. Data are plotted against the intensity of the second stimulus. Error bars are standard deviations. For T2 and P3 no values are given for the lowest intensity of the second stimulus, since it was not clearly distinguishable in the single subjects.
When two magnetic stimuli (large S1, S2 just above active motor threshold) were given at an interstimulus interval of 1 ms in the isometrically contracting ADM, strong MEP facilitation occurred in both subjects tested (Fig. 4A). The onset of facilitation, i.e. when the MEP elicited by paired TMS exceeded the sum of the MEPs elicited by S1 and S2 alone, was some 1.5 ms later than the onset of the MEP elicited by S2 alone (Fig. 4A). In contrast, if S2 was substituted by anodal (Fig. 4B) or cathodal TES (Fig. 4D), no consistent MEP facilitation occurred in the active ADM at an interstimulus interval of 1 ms. In order to correct for the difference in onset latencies of the MEP produced by TMS and anodal TES, the interstimulus interval was adjusted to 2.7 ms in both subjects. Again, no MEP facilitation was observed (Fig. 4C).
Figure 4. Motor-evoked potentials to paired transcranial magnetic or combined magnetic and electrical stimulation in the active ADM.
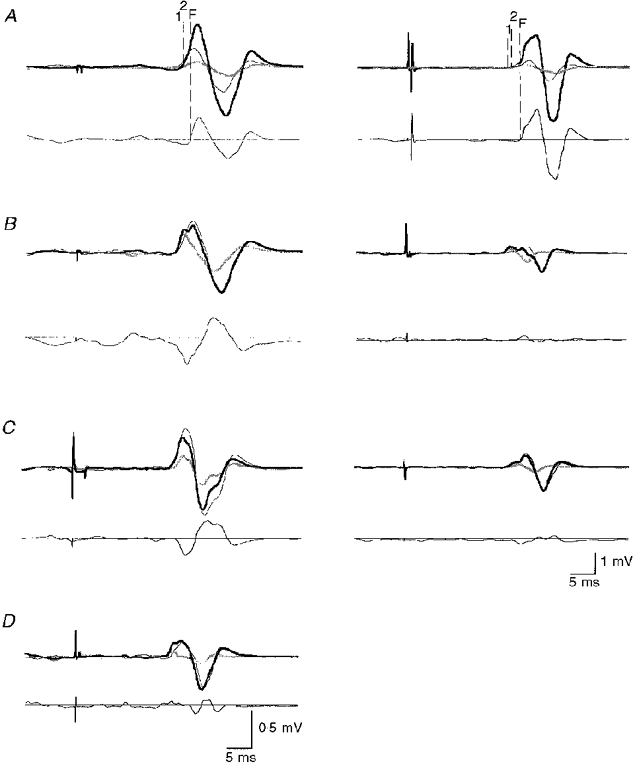
Upper parts of the figures show MEP recordings from 2 subjects (left and right column). The first stimulus (S1) was suprathreshold TMS; the second stimulus (S2) was slightly above threshold, and TMS in A, anodal TES in B and C, or cathodal TES in D (only one subject, since the threshold for cathodal TES exceeded the maximum stimulator output in the other subject). The interstimulus interval was 1 ms in A, B and D, but it was 2.7 ms in C to correct for the difference of 1.7 ms in onset latency of the MEPs produced by TMS and anodal TES. MEPs to single stimulation are shown as thin black and thick grey lines for S1 and S2, respectively. The MEPs to paired stimulation are the thick black lines. All recordings are averages of 10 trials. The vertical dashed lines indicate the onset of the MEP to S1 (1), the onset of the MEP to S2 (2), and the time when the MEP to paired stimulation exceeded the sum of the MEPs elicited by single stimulation (facilitation, F). Lower parts of the figures show the calculated difference between the MEP to paired stimulation and the algebraic sum of the MEPs to single stimulation. The horizontal lines indicate the zero level. Note that MEP facilitation occurred only with paired magnetic stimulation, not with a combination of TMS and TES.
Figure 5 shows the change in MEP size across interstimulus intervals from 0.5 to 5.1 ms after paired TMS in the active ADM. At short intervals (1.1-1.7 ms) an even stronger MEP facilitation occurred when compared with the resting condition (cf. Fig. 1B). A mismatch in stimulus intensities was unlikely to account for this result, since the intensity of S1 was adjusted for both conditions so as to elicit a MEP of about 1 mV on its own, and S2 was set to 90 % of active motor threshold and 90 % of resting motor threshold in the active and resting conditions, respectively. Another result from this experiment was that the second and third peaks of MEP facilitation disappeared during voluntary contraction (Fig. 5).
Figure 5. Motor-evoked potential facilitation by paired transcranial magnetic stimulation in the active ADM.
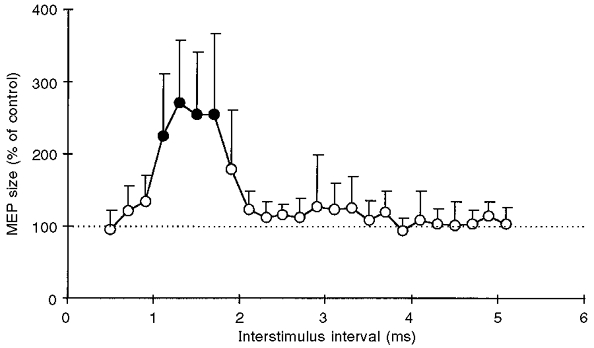
Data are means from the same 5 subjects as in Fig. 1B, tested 3 times each with the ADM tonically active. The first stimulus was set to produce a control response of about 1 mV. The second stimulus was set to 90 % of active motor threshold. •, indicates motor responses significantly different from control (P < 0.05, Bonferroni-corrected multiple t tests). Otherwise, same arrangement and conventions as in Fig. 1B
DISCUSSION
Paired TMS of the human motor cortex results in a succession of at least three facilitatory MEP peaks if the first stimulus (S1) is suprathreshold and the second stimulus (S2) subthreshold for eliciting a MEP in the ADM muscle. The facilitatory interstimulus intervals occur at about 1.3, 2.7 and 4.2 ms. The facilitatory MEP peaks are separated by troughs at interstimulus intervals of about 2.0 and 3.3 ms where S2 has no effect on the size of the contol MEP produced by S1 alone.
The site of interaction between the first and second stimuli
From epidural-evoked spinal cord potential recordings, it is well established that a suprathreshold magnetic stimulus over the hand area of the human motor cortex can produce multiple corticospinal volleys compatible with an initial D wave followed by several I waves (e.g. Burke et al. 1993; Fujiki et al. 1996; Nakamura et al. 1997). The latencies of about 1.5 ms between MEP peaks observed in the present experiments are closely compatible with the latencies between successive I waves reported in the epidural spinal recording studies. Therefore, we propose that our findings should be explained on the basis of D-I discharges.
The first question which arises is whether the facilitatory interaction between S1 and S2 in the present experiments took place primarily at the cortical or spinal level, or both. While there exists a large body of literature on the integrative capacity of the spinal motoneurone (for extensive review, see Baldissera et al. 1981), much less is known about the corticospinal neurone or motor cortex interneurones. Although a summation of the complex corticospinal volley after suprathreshold motor cortex stimulation certainly occurs at the level of the spinal motoneurone, we will argue below that our findings are evidence for a primarily cortical interaction between S1 and S2, at least for the first MEP peak. A cortical interaction hypothesis would imply that a given corticospinal neurone is capable of firing repetitively at a rate high enough to follow the I wave frequency. Indeed, the human corticospinal tract can follow stimulation rates higher than 500 Hz when directly activated (Katayama et al. 1988; Fujiki et al. 1996), and single-axon recordings in the monkey demonstrated that a single corticospinal axon can produce multiple I waves (Kernell & Chien-Ping, 1967; Edgley et al. 1997). It is also known that a single corticospinal neurone can give rise to an increasing number of I waves as a function of stimulus intensity (Edgley et al. 1997). The cortical facilitation hypothesis would then predict an increased probability for a corticospinal neurone to give rise to an I wave in response to a suprathreshold first stimulus if the subthreshold second stimulus was delivered at a facilitatory interstimulus interval.
The following of our findings support the cortical facilitation hypothesis. A small and delayed facilitatory peak was already present even when the intensity of S1 was far below motor threshold (70 % of resting motor threshold, Fig. 2). It is known from previous studies that such low intensity stimulation has no effect on spinal motoneurone excitability as measured with H reflexes (Kujirai et al. 1993; Ziemann et al. 1996). This suggests that these low intensities are below the threshold for producing a significant corticospinal volley pointing to a supraspinal, probably cortical, level for the interaction with S2.
Another argument against a significant spinal interaction of the effects of the two magnetic stimuli comes from the experiments on varying the intensity of S2 with the intensity of S1 fixed above threshold (Fig. 3). If there was a spinal interaction, the timing of the peaks should have decreased to shorter interstimulus intervals with increasing intensity of S2, since we know from several epidural spinal recording studies that I waves advance to slightly shorter onset latencies with increasing stimulus intensity (Fig. 3 in Kitagawa et al. 1995; Fig. 1 in Nakamura et al. 1997; Fig. 1 in Edgley et al. 1997). However, the timing of the peaks and troughs in our study remained constant irrespective of the intensity of S2 (Fig. 3A and B). If we assume that the MEP facilitation after paired TMS was due to a facilitatory interaction between I waves (for evidence, see below), then this finding indicates that the interaction took place in the cortex at the very structures where I waves are generated, i.e the excitatory interneurones and the corticospinal neurones on which they project.
A final argument against a spinal interaction between the effects of two magnetic stimuli comes from the experiments shown in Fig. 4. When two magnetic stimuli were given at an interstimulus interval of 1 ms while the ADM was tonically active, both subjects showed a marked MEP facilitation (Fig. 4A). If the second stimulus was replaced by TES and the interstimulus interval was changed to 2.7 ms, no MEP facilitation occurred (Fig. 4C). In the active muscle, anodal or cathodal TES at just above threshold produces predominantly a D wave through direct activation of corticospinal neurones at or close to the initial axon segment, but no I waves (e.g. Day et al. 1989). The adjustment of the interstimulus interval was made to correct for the difference in the onset latency of MEPs produced by TMS and anodal TES, which was 1.7 ms in both subjects. With this adjustment, the D wave from anodal TES should arrive at the spinal motoneurone pool approximately 1 ms later than the first I wave from the magnetic first stimulus. This would be equivalent to the spinal delay of the first I waves in the paired TMS experiments in Fig. 4A. Therefore, the lack of MEP facilitation with TMS-TES at an interstimulus interval of 2.7 ms is strong evidence against a significant spinal contribution to the facilitatory interaction between the effects of two magnetic stimuli.
The mode of interaction between the first and second stimuli
Recent epidural spinal recordings were made in conscious humans in the absence of anaesthetic agents that suppress the generation of I waves (Di Lazzaro et al. 1997; Nakamura et al. 1997). In these studies, it was shown that if the intensity of a single magnetic stimulus was gradually increased, first an early I wave appeared, at higher intensities later I waves emerged, and at still higher intensities a D wave was detected. This suggests that an interaction between I waves and a D wave was unlikely in the present experiments when a subthreshold S2 was used.
As further evidence against a significant contribution of D waves, we found that no consistent MEP facilitation occurred in the active ADM when suprathreshold TMS was followed by anodal or cathodal TES at an interstimulus interval of 1 ms (Fig. 4B and D). In contrast to the experiments shown in Fig. 4C (for discussion, see above), in these experiments no adjustment was made for the difference in MEP onset latency between TMS and TES. Therefore, the absence of MEP facilitation with TMS-TES points against a significant contribution of D waves at any level along the corticospinal system to the marked facilitatory interaction between the effects of two magnetic stimuli given at the same interstimulus interval of 1 ms (Fig. 4A).
The experiments illustrated in Fig. 4A allow one further conclusion. At MEP onset, voluntary muscle activation eliminates the time required for summation of excitatory postsynaptic potentials (EPSPs) in order to bring the spinal motoneurone above firing threshold. If it is assumed that TMS mainly activates a monosynaptic corticospinal projection to spinal motoneurones supplying the ADM muscle, then any difference in MEP onset latency can be ascribed to processes in the motor cortex. A facilitatory interaction between D and I waves at the corticospinal neurone would have led to an onset of MEP facilitation earlier than the onset of the MEP produced by S2 and about equal to the onset of the MEP from S1. However, the onset of MEP facilitation was later by some 1.5 ms than the onset of the MEP elicited by S2 (Fig. 4A). This indicates that the interaction between two magnetic stimuli took place at or ‘upstream’ from the corticospinal neurone and involved I but not D waves.
The second and third MEP peaks were not studied in the same detail as the first peak. However, the consistency of the MEP peak latencies (Fig. 3A and B) makes it very likely that these peaks also originated from (later) I wave interactions in the motor cortex.
The exact nature of the facilitatory I wave interaction cannot be elucidated further with the present techniques. One possibility is that in response to a large sustained depolarization the corticospinal neurone discharges repetitively as a product of its intrinsic membrane properties (Creutzfeldt et al. 1964; for review, see Phillips, 1987). This model, which explains both the observed MEP peaks and troughs, predicts that S2 has a visible effect on MEP size only (provided that S2 < S1), if cortico-cortical discharges produced by S2 arrive at the corticospinal neurone during the epochs of increased firing probability as set by S1. The late onset of the MEP facilitation at 1.5 ms after the onset of the MEP elicited by S2 alone (Fig. 4) could be a consequence of the relatively long refractory period of cortico-cortical fibres (Amassian et al. 1998). Another hypothesis posits that the repetitive discharge of corticospinal neurones at I wave frequency is a product of multiple EPSPs impinging on the corticospinal neurone through different chains of cortico-cortical neurones (Amassian et al. 1987; Day et al. 1989). A difficulty with this model is, however, that to explain the troughs between the MEP peaks may require the assumption of a complex sequence of alternating EPSPs and inhibitory postsynaptic potentials (IPSPs) at the corticospinal neurone. While cortical surface stimulation in the monkey can lead to a single EPSP truncated by a following IPSP (Ghosh & Porter, 1988), sequences of EPSPs and IPSPs have not been reported.
Paired TMS during voluntary contraction of the ADM
It remains an open question whether the increase in the magnitude of the first MEP peak (Fig. 5) was caused by spinal or cortical mechanisms, or both. The available literature on MEP facilitation during voluntary contraction is conflicting, supporting either predominantly spinal (Maertens de Noordhout et al. 1992; Ugawa et al. 1995) or cortical (Lemon et al. 1995; Baker et al. 1995) mechanisms.
The disappearance of the second and third MEP peaks in the paired TMS protocol with the ADM tonically active (Fig. 5) may be explained through spinal inhibitory mechanisms such as after-hyperpolarization and recurrent inhibition, which become more effective as a function of the level of preceding depolarization or the amount of synchronous activation of the spinal motoneurone (Ito & Oshima, 1962; Hultborn et al. 1979).
In conclusion, we have provided evidence that paired-pulse TMS can be used to demonstrate I waves non-invasively in intact humans. We argue that the facilitatory interaction between the two stimuli occurs predominantly at the level of the motor cortex. This technique may be applied to investigate alterations in I wave production as they may occur in human brain disease and under various other experimental conditions.
Acknowledgments
We are very grateful to Dr John Rothwell and Professor Vahe Amassian for a number of excellent discussions. Parts of this study have been published in abstract form (Tergau et al. 1997).
References
- Amassian VE, Rothwell JC, Cracco RQ, Maccabee PJ, Vergara M, Hassan N, Eberle L. What is excited by near-threshold twin magnetic stimuli over human cerebral cortex. The Journal of Physiology. 1998;506.P:122P. [Google Scholar]
- Amassian VE, Stewart M, Quirk GJ, Rosenthal JL. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74–93. [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. Task-related variation in corticospinal output evoked by transcranial magnetic stimulation in the macaque monkey. The Journal of Physiology. 1995;488:795–801. doi: 10.1113/jphysiol.1995.sp021011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Brookhart JM, Mountcastle VB, editors. Handbook of Physiology, section 1, The Nervous System. Vol. 2. Baltimore: Waverly Press, Inc.; 1981. pp. 509–596. [Google Scholar]
- Boniface SJ, Mills KR, Schubert M. Responses of single spinal motoneurons to magnetic brain stimulation in healthy subjects and patients with multiple sclerosis. Brain. 1991;114:643–662. doi: 10.1093/brain/114.1.643. [DOI] [PubMed] [Google Scholar]
- Boyd SG, Rothwell JC, Cowan JM, Webb PJ, Morley T, Asselman P, Marsden CD. A method of monitoring function in cortico-spinal pathways during scoliosis surgery with a note on motor conduction velocities. Journal of Neurology, Neurosurgery and Psychiatry. 1986;49:251–257. doi: 10.1136/jnnp.49.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hicks R, Gandevia SC, Stephen J, Woodforth I, Crawford M. Direct comparison of cortico-spinal volleys in human subjects to transcranial magnetic and electrical stimulation. The Journal of Physiology. 1993;470:383–393. doi: 10.1113/jphysiol.1993.sp019864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan JM, Day BL, Marsden C, Rothwell JC. The effect of percutaneous motor cortex stimulation on H reflexes in muscles of the arm and leg in intact man. The Journal of Physiology. 1986;377:333–347. doi: 10.1113/jphysiol.1986.sp016190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt OD, Lux HD, Nacimiento AC. Intracelluläre reizung corticaler nervenzellen. Pflügers Archiv. 1964;281:129–151. [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. The Journal of Physiology. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell J. Effect of voluntary contraction on the threshold and size of cortico-spinal volleys evoked by transcranial magnetic or electric stimulation in conscious man. Electroencephalography and Clinical Neurophysiology. 1997;103:193. [Google Scholar]
- Edgley SA, Eyre JA, Lemon RN, Miller S. Comparison of activation of cortico-spinal neurons and spinal motor neurons by magnetic and electrical transcranial stimulation in the lumbosacral cord of the anaesthetized monkey. Brain. 1997;120:839–853. doi: 10.1093/brain/120.5.839. [DOI] [PubMed] [Google Scholar]
- Fujiki M, Isono M, Hori S, Ueno S. Cortico-spinal direct response to transcranial magnetic stimulation in humans. Electroencephalography and Clinical Neurophysiology. 1996;101:48–57. doi: 10.1016/0013-4694(95)00122-0. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Porter R. Corticocortical synaptic influences on morphologically identified pyramidal neurones in the motor cortex of the monkey. The Journal of Physiology. 1988;400:617–629. doi: 10.1113/jphysiol.1988.sp017139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks R, Burke D, Stephen J, Woodforth I, Crawford M. Cortico-spinal volleys evoked by electrical stimulation of human motor cortex after withdrawal of volatile anaesthetics. The Journal of Physiology. 1992;456:393–404. doi: 10.1113/jphysiol.1992.sp019342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Pierroty-Deseilligny E, Wigström H. Recurrent inhibition and afterhyperpolarization following motoneuronal discharge in the cat. The Journal of Physiology. 1979;297:253–266. doi: 10.1113/jphysiol.1979.sp013038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Oshima T. Temporal summation of after-hyperpolarization following a motoneurone spike. Nature. 1962;195:910–911. [Google Scholar]
- Kaneko K, Kawai S, Fuchigami Y, Morita H, Ofuji A. The effect of current direction induced by transcranial magnetic stimulation on the cortico-spinal excitability in human brain. Electroencephalography and Clinical Neurophysiology. 1996;101:478–482. doi: 10.1016/s0013-4694(96)96021-x. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Tsubokawa T, Maejima S, Hirayama T, Yamamoto T. Cortico-spinal direct response in humans: identification of the motor cortex during intracranial surgery under general anaesthesia. Journal of Neurology, Neurosurgery and Psychiatry. 1988;51:50–59. doi: 10.1136/jnnp.51.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D, Chien-Ping WU. Responses of the pyramidal tract to stimulation of the baboon's motor cortex. The Journal of Physiology. 1967;191:653–672. doi: 10.1113/jphysiol.1967.sp008273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa H, Nakamura H, Kawaguchi Y, Tsuji H, Satone T, Takano H, Nakatoh S. Magnetic-evoked compound muscle action potential neuromonitoring in spine surgery. Spine. 1995;20:2233–2239. doi: 10.1097/00007632-199510001-00010. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. The Journal of Physiology. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN, Johansson RS, Westling G. Corticospinal control during reach, grasp, and precision lift in man. Journal of Neuroscience. 1995;15:6145–6156. doi: 10.1523/JNEUROSCI.15-09-06145.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens de Noordhout A, Pepin JL, Gerard P, Delwaide PJ. Facilitation of responses to motor cortex stimulation: effects of isometric voluntary contraction. Annals of Neurology. 1992;32:365–370. doi: 10.1002/ana.410320310. [DOI] [PubMed] [Google Scholar]
- Mazzocchio R, Rothwell JC, Day BL, Thompson PD. Effect of tonic voluntary activity on the excitability of human motor cortex. The Journal of Physiology. 1994;474:261–267. doi: 10.1113/jphysiol.1994.sp020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri B, Wassermann EM, Manganotti P, Ikoma K, Samii A, Hallett M. Cortical modulation of spinal excitability: an F-wave study. Electroencephalography and Clinical Neurophysiology. 1996;101:16–24. doi: 10.1016/0013-4694(95)00164-6. [DOI] [PubMed] [Google Scholar]
- Mills KR. Magnetic brain stimulation: a tool to explore the action of the motor cortex on single human spinal motoneurones. Trends in Neurosciences. 1991;14:401–405. doi: 10.1016/0166-2236(91)90029-t. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Direct and indirect activation of human cortico-spinal neurons by transcranial magnetic and electrical stimulation. Neuroscience Letters. 1996;210:45–48. doi: 10.1016/0304-3940(96)12659-8. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. The Journal of Physiology. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton HD, Amassian VE. Single- and multiple-unit analysis of cortical stage of pyramidal tract activation. Journal of Neurophysiology. 1954;17:345–363. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- Phillips CG. Epicortical electrical mapping of motor areas in primates. In: Bock G, O'Connor M, Marsh J, editors. Motor Areas of Cerebral Cortex. London: John Wiley; 1987. pp. 5–20. [DOI] [PubMed] [Google Scholar]
- Rothwell J, Burke D, Hicks R, Stephen J, Woodforth I, Crawford M. Transcranial electrical stimulation of the motor cortex in man: further evidence for the site of activation. The Journal of Physiology. 1994;481:243–250. doi: 10.1113/jphysiol.1994.sp020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tergau F, Ziemann U, Hildebrandt J, Paulus W. Demonstration of I waves by paired transcranial magnetic stimulation (TMS) in humans. Electroencephalography and Clinical Neurophysiology. 1997;103:192. doi: 10.1016/s0924-980x(98)00023-x. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalography and Clinical Neurophysiology. 1996;101:263–272. doi: 10.1016/0924-980x(96)95664-7. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Terao Y, Hanajima R, Sakai K, Kanazawa I. Facilitatory effect of tonic voluntary contraction on responses to motor cortex stimulation. Electroencephalography and Clinical Neurophysiology. 1995;97:451–454. doi: 10.1016/0924-980x(95)00214-6. [DOI] [PubMed] [Google Scholar]
- van der Linden C, Bruggeman R. Multiple descending cortico-spinal volleys demonstrated by changes of the wrist flexor H-reflex to magnetic motor cortex stimulation in intact human subjects. Muscle and Nerve. 1993;16:374–378. doi: 10.1002/mus.880160406. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. The Journal of Physiology. 1996;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]


