Abstract
Previous studies demonstrated that both ventilatory and phrenic nerve responses to acute hypoxia are greatly attenuated in adult rats (3–5 months old) previously exposed to 1 month of perinatal hyperoxia (60% O2; perinatal treated rats). The present study tested the hypothesis that this functional impairment recovers spontaneously with advancing age in perinatal treated rats.
Hypoxia-induced chemoreflexes were examined by measuring integrated phrenic responses to strictly controlled isocapnic hypoxia in urethane-anaesthetized, vagotomized, paralysed and ventilated rats at different ages.
At 50 mmHg Pa,O2 (arterial O2 partial pressure), the hypoxia-induced increase in minute phrenic activity was significantly attenuated in both 3- to 5-month-old (166 ± 15% of baseline) and 6-month-old (130 ± 17%) perinatal treated rats, relative to 3- to 6-month-old, untreated control rats (279 ± 28%; both P < 0.05). However, at 40 mmHg Pa,O2, the hypoxic minute phrenic activity response was attenuated only in 3- to 5-month-old (154 ± 33%), but not 6-month-old (232 ± 33%) perinatal treated rats versus control rats (293 ± 30%).
The minute phrenic activity response to hypoxia was not significantly different between geriatric perinatal treated rats (14–15 months) and untreated geriatric control rats at either 50 mmHg (treated: 250 ± 20%versus control: 274 ± 23%) or 40 mmHg Pa,O2 (treated: 292 ± 19%versus control: 315 ± 36%).
These data suggest that partial spontaneous recovery may occur in 6-month-old perinatal treated rats and that full recovery occurs by 15 months of age.
Most neural systems continue profound development after birth, in which adequate sensory activity during early critical periods is often necessary for normal development (cf. Kandel & Jessell, 1991). To investigate the role of such developmental plasticity in the hypoxic ventilatory control system, we developed a model of reversible sensory suppression from peripheral arterial chemoreceptors by exposing rats to moderate hyperoxia (60% O2) during the first month of life (perinatal treated rats). In these perinatal treated rats, both awake ventilatory (Ling et al. 1996) and anaesthetized phrenic nerve responses (Ling et al. 1997b) to acute hypoxia were greatly attenuated several months after the perinatal hyperoxia had ended. Hypercapnic ventilatory responses in perinatal treated rats were unchanged, suggesting that the functional deficit is unique to hypoxia-induced chemoreflexes (Ling et al. 1996). Since functional impairment of ventilatory or phrenic responses to hypoxia was not observed in rats exposed to hyperoxia as adults, the hyperoxia-induced deficit is both persistent and unique to development (Ling et al. 1996, 1997b). The functional impairment results from a persistent deficit in carotid body chemotransduction (Ling et al. 1997c) rather than in the central integration of carotid chemoafferent inputs (Ling et al. 1997a). In association with these physiological findings, a persistent loss of carotid body volume and cell number, and a decrease in the number of axons within the carotid sinus nerve, is observed following perinatal hyperoxia (Erickson et al. 1998).
Functional impairment following sensory deprivation can be either permanent or partially reversible, depending on the duration and timing of the sensory deprivation (Movshon, 1976; Antonini & Stryker, 1996). Thus, our purpose in the present study was to test the hypothesis that spontaneous functional recovery of the hypoxic ventilatory response occurs with advancing age in perinatal treated rats. Hypoxic phrenic responses were measured in perinatal treated rats at different ages along with size, sex and age matched (untreated) control rats. Such information is of significance since it will shed light on whether or not this form of developmental plasticity is permanent, or if it in turn exhibits a degree of reversibility.
A portion of these results has appeared in abstract form (Ling et al. 1997d).
METHODS
Experimental groups
Experiments were conducted on adult male Sprague-Dawley rats (Harlan Sprague-Dawley Inc., Madison, WI, USA). There were two groups of untreated control rats: young adult (n = 7; 480 ± 21 g; 3–6 months of age) and geriatric control rats (n = 4, from three different litters; 625 ± 25 g; 14–15 months of age). Perinatal treated rats were those previously exposed to moderate ambient hyperoxia (60% O2) from approximately 2 days before birth through their first 28 postnatal days (cf. Ling et al. 1996), and were studied in the following three age groups: 3–5 months old (n = 7; 440 ± 14 g), 6 months old (n = 4; 510 ± 12 g) and geriatric perinatal treated rats (14–15 months old; n = 5; 635 ± 7 g; from four different litters).
Experimental preparation and protocols
Anaesthesia was induced with isoflurane in a closed box, and maintained with isoflurane, initially by nose cone and then through a tracheal cannula (2.5-3.0% in 50% O2, balance N2). An intravenous catheter was placed in a femoral vein to deliver drugs, and the rats were slowly converted to urethane anaesthesia (1.3-1.6 g kg−1, i.v.). The adequacy of anaesthesia was assessed regularly by testing corneal reflexes and/or blood pressure responses to toe pinch throughout an experiment; supplemental urethane was administered as necessary. At the end of acute experiments, the animals were killed by an overdose of urethane. These animal procedures were approved by the animal care and use committee of the University of Wisconsin-Madison. An arterial catheter was placed in the femoral artery to continuously monitor blood pressure (Statham Pressure Transducer, P23-id) and to withdraw blood samples to determine blood gases and pH (ABL-330; Radiometer, Copenhagen, Denmark). Animals were vagotomized, paralysed (pancuronium bromide, 2.5 mg kg−1) and ventilated (Harvard Rodent Respirator).
The left phrenic nerve was isolated via a dorsal approach, cut distally, desheathed and prepared for recording with a bipolar silver wire electrode. Nerve activity was amplified (×10 000; CWE BMA 831; Ardmore, PA, USA), band-pass filtered (100 Hz to 5 kHz) and integrated (Paynter filter CWE 821; time constant, 100 ms). The integrated phrenic signal was digitized (Scientific Solutions Inc., Lab Master DMA; Solon, OH, USA) and processed with computer software developed in our laboratory. This software determined the amplitude and timing of integrated phrenic bursts, from which the minute phrenic activity was calculated.
Inspired gases were 50% O2 (balance N2) in baseline conditions to improve the rat's tolerance of experimental stresses, and to prolong the viability of the preparation. Rectal temperature was monitored and maintained near 38°C with a heated table. Baseline phrenic activity was set at ∼30% of maximal hypercapnic phrenic discharge by manipulating the inspired CO2 and respiratory pump rate and/or volume while monitoring end-tidal PCO2 levels. It is important to standardize the magnitude of baseline phrenic motor outflow in these experiments due to the fact that the input-output of phrenic responses to chemoreceptor activation is not linear (cf. Eldridge et al. 1981). Hypercapnic ventilatory responses are normal in awake perinatal treated rats (Ling et al. 1996), providing a common standard to compare hypoxic phrenic responses between treated and control rats.
Chemoreflexes were examined by recording integrated phrenic nerve activity during baseline (inspired O2 fraction (FI,O2) = 0.50; arterial O2 partial pressure (Pa,O2) > 180 mmHg; arterial CO2 partial pressure (Pa,CO2), 40–55 mmHg), at the plateau (3–5 min) of two levels of isocapnic hypoxia (40 and 50 mmHg Pa,O2) and during hypercapnia (end-tidal PCO2, 90–95 mmHg) with intervals of 20–30 min between measurements. Arterial blood gases (CO2 and O2) were determined throughout an experiment (ABL-330; Radiometer) and levels of hypoxaemia were kept within 4 mmHg Pa,O2 of target levels in each animal by adjusting the inspired O2 fraction. End-tidal PCO2 was monitored using a flow-through capnograph (Novametrix; Wallingford, CT, USA) with sufficient response time (< 75 ms) to measure rat end-tidal PCO2. Values of end-tidal PCO2 obtained from this capnograph closely approximated Pa,CO2 in most rats (usually within 1–2 mmHg). If deviations in end-tidal PCO2 or Pa,CO2 from isocapnic conditions were noted, corrections were made by adjusting the inspired CO2 fraction (FI,CO2), thus assuring that Pa,CO2 was within 2 mmHg of the baseline value (Table 1). It was usually necessary to elevate FI,CO2 (0.007-0.012) to maintain isocapnia during hypoxia, indicating that CO2 flux at the lungs had decreased (either due to decreased metabolic rate or a consistent blood pressure drop).
Table 1.
Blood gas values during isocapnic hypoxia in untreated control and perinatal treated rats
| Group | Baseline (mmHg) | Moderate hypoxia (mmHg) | Mild hypoxia (mmHg) | |
|---|---|---|---|---|
| Pa,O2 | 3- to 5-month-old treated (7) | 221 ± 11.3 | 38.7 ± 1.1 | 47.7 ± 0.9 |
| 6-month-old treated (4) | 232 ± 12.0 | 40.9 ± 0.2 | 50.5 ± 0.4 | |
| 3- to 6-month-old control (7) | 224 ± 11.0 | 40.7 ± 1.3 | 49.6 ± 1.6 | |
| Geriatric treated (5) | 211 ± 7.9 | 40.5 ± 1.0 | 48.4 ± 0.9 |
| Change from baseline | ||||
|---|---|---|---|---|
| Pa,CO2 | 3- to 5-month-old treated (7) | 44.8 ± 1.6 | 0.3 ± 0.9 | −0.4 ± 1.1 |
| 6-month-old treated (4) | 50.0 ± 1.1 | −0.6 ± 0.4 | −0.6 ± 0.4 | |
| 3- to 6-month-old control (7) | 47.9 ± 1.0 | −1.2 ± 0.5 | −1.3 ± 0.7 | |
| Geriatric treated (5) | 46.2 ± 2.8 | 0.2 ± 0.6 | −0.3 ± 0.7 | |
| Geriatric control (4) | 46.5 ± 1.6 | −0.9 ± 1.2 | −0.3 ± 0.8 | |
Data are means ±s.e.m. Number of rats in parentheses. Note that mean hypoxic Pa,O2 (arterial O2 partial pressure) was very close to the designated 40 mmHg (moderate hypoxia) and 50 mmHg (mild hypoxia) in all 5 groups. Mean Pa,CO2 during hypoxia was not significantly changed from baseline in any group (P > 0.05).
More detailed descriptions of this experimental preparation and protocol are available in a published paper (Ling et al. 1997b).
Data analysis
A randomized, blinded design was used to conceal the identity of rats (treated versus control at a given age) from the investigator. Phrenic activity was averaged over fifty to sixty bursts in each condition. Variables determined included: phrenic burst frequency (f, bursts min−1), peak amplitude of integrated phrenic activity (∫Phr) and their product, the minute phrenic activity (∫Phr ×f). Changes from baseline in ∫Phr and ∫Phr ×f were normalized as a percentage of the baseline phrenic nerve activity (%baseline) and as a percentage of the phrenic activity during hypercapnia (%maximum). The %maximum normalization obviates several potential normalization artifacts when comparing neurograms under different conditions within an animal, or the same neurogram in different animals (cf. Fregosi & Mitchell, 1994). However, in this paper, all data are expressed as a% baseline since the results were similar with both normalization methods. A two-way ANOVA with repeated measures (SigmaStat Version 2.0, Jandel Corporation, San Rafael, CA, USA), followed by Bonferroni t tests, was used to test the significance of differences in variables (Δf, Δ∫Phr and Δ(∫Phr ×f)) between rat groups. Differences were considered significant at the P < 0.05 level. All data are presented as mean values ± 1 s.e.m.
RESULTS
The magnitude of attenuation in hypoxic phrenic responses decreased slowly with advancing age in perinatal treated rats. At 50 mmHg Pa,O2, hypoxia-induced increases in minute phrenic activity (Δ(∫Phr ×f)) were attenuated in both 3- to 5-month-old (166 ± 15% of baseline) and 6-month-old (130 ± 17%) perinatal treated rats, relative to young adult, untreated control rats (279 ± 28%; 3–6 months; both P < 0.05; Fig. 1). However, at 40 mmHg Pa,O2, Δ(∫Phr ×f) was attenuated only in 3- to 5- (154 ± 33%) but not 6-month-old (232 ± 33%) perinatal treated rats versus control (293 ± 30%; Fig. 1). At 50 mmHg Pa,O2, hypoxia-induced increases in the peak amplitude of integrated phrenic activity (Δ∫Phr) were also significantly attenuated in both 3- to 5-month-old (88 ± 9%) and 6-month-old (86 ± 18%) perinatal treated rats versus control rats (158 ± 16%). In contrast, at 40 mmHg Pa,O2, Δ∫Phr was attenuated in 3- to 5-month-old (81 ± 19%), but not 6-month-old (146 ± 37%) perinatal treated rats versus control (154 ± 20%; Fig. 1). Although it appeared that hypoxia-induced increases in phrenic burst frequency (Δf) were attenuated in both 3- to 5-month-old and 6-month-old perinatal treated rats, these differences were not statistically significant (Fig. 1).
Figure 1. Hypoxic phrenic responses in untreated control and perinatal treated rats.
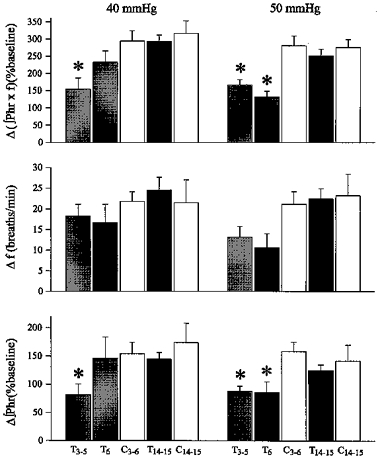
Data were obtained from 3- to 5-month-old (T3–5; n = 7), 6-month-old (T6; n = 4) and geriatric (T14–15; 14–15 months; n = 5) perinatal treated rats, compared with young adult (C3–6; 3–6 months; n = 7) and geriatric untreated control rats (C14–15; 14–15 months; n = 4). Mean hypoxia-induced increases from baseline in minute phrenic activity (Δ(∫Phr ×f) (%baseline)), phrenic burst frequency (Δf (breaths min−1)) and peak amplitude of integrated phrenic activity (Δ∫Phr (%baseline)) were measured at 2 isocapnic hypoxic levels (Pa,O2: 40 and 50 mmHg) and expressed as means ±s.e.m.* Significant difference from the untreated control group of similar age at the same hypoxic level (P < 0.05).
Unlike literature accounts of hypoxic ventilatory responses in awake geriatric rats (Fukuda, 1992), hypoxic phrenic responses were not different between young adult and geriatric control rats in the present study. Δ(∫Phr ×f), Δf and Δ∫Phr were all similar between young adult (3–6 months of age; n = 7) and geriatric untreated control rats (14–15 months; n = 4) at both 40 and 50 mmHg Pa,O2 (Fig. 1). Similarly, Δ(∫Phr ×f) was not significantly different between geriatric perinatal treated rats (14–15 months) and geriatric control rats at either 50 mmHg (treated: 250 ± 20%versus control: 274 ± 23%) or 40 mmHg Pa,O2 (treated: 292 ± 19%versus control: 315 ± 36%; both P > 0.05). Neither Δf nor Δ∫Phr responses to hypoxia were affected by perinatal hyperoxia in the geriatric groups (Fig. 1).
Mean Pa,CO2 levels were not significantly different among experimental conditions within any rat group. Mean Pa,O2 and Pa,CO2 levels were not significantly different among experimental groups in any given condition (Table 1).
DISCUSSION
The main finding of the present study is the observation that the attenuated hypoxic phrenic responses at both 40 and 50 mmHg Pa,O2 returned to normal levels when perinatal treated rats became very old (15 months), suggesting that although the perinatal hyperoxia-induced impairment in functional performance of the hypoxic ventilatory control system is persistent, it is not permanent and can fully recover with advancing age. Hypoxic phrenic responses were attenuated at both 40 and 50 mmHg Pa,O2 in 3- to 5-month-old, but only at 50 mmHg Pa,O2 in 6-month-old perinatal treated rats, suggesting a degree of partial recovery already by 6 months of age.
In awake perinatal treated rats (3- to 5-months-old), although minute ventilatory response to moderate hypoxia (∼48 mmHg Pa,O2) is greatly attenuated (∼1/3 of control), more severe hypoxia (41 mmHg) can still generate a bigger ventilatory response (Ling et al. 1996). Thus, the ability to respond to hypoxaemia is not totally abolished in perinatal treated rats; the hypoxic ventilatory response might only be shifted to lower levels of Pa,O2. On the other hand, responses to 40 mmHg Pa,O2 may have been close to maximal levels since we observed on multiple occasions that hypoxic responses do not become bigger when hypoxaemic levels are further reduced. In the present study, hypoxic phrenic responses were attenuated at 50, but not 40 mmHg Pa,O2 in 6-month-old perinatal treated rats, suggesting that recovery is gradual and might first occur in more severe hypoxic responses.
Functional recovery from developmental deficits also exists in other neural systems. For example, although one of the hallmarks of ocular dominance plasticity in cats is essentially permanent functional impairment following 7–12 weeks of monocular deprivation (Hubel & Wiesel, 1970; Wiesel, 1982), at least partial recovery is observed following sensory deprivation of shorter durations (Movshon, 1976; Antonini & Stryker, 1996). Thus, the persistence of functional impairment following sensory deprivation/suppression during development depends on the duration and timing of the insult.
In the present study, functional performance of the hypoxic ventilatory control system was assessed by measuring integrated phrenic responses to acute, isocapnic hypoxia in anaesthetized rats. This approach allowed us to examine directly the neural component of the O2 chemoreflex, by-passing effects from possible changes in respiratory mechanics. The preparation also allowed us to readily control blood gases throughout an experiment, obviating effects from possible changes in gas exchange. Blood gas responses to hypoxia are different in perinatal treated rats versus untreated control rats at the same FI,O2(Ling et al. 1996). Therefore, in the present study, to compare similar chemoreceptor stimuli we chose Pa,O2 instead of FI,O2as our hypoxic index and also kept strict isocapnia during hypoxia. These measures, however, limited our ability to run more tests at multiple hypoxic levels in order to plot a response curve. Thus, we focused on two sensitive levels (40 and 50 mmHg Pa,O2) to be consistent with the protocol in our previous studies (Ling et al. 1997b).
In contrast to perinatal treated rats, integrated phrenic responses to acute hypoxia did not change with advancing age in untreated control rats. In some respects, these data appeared to be inconsistent with reported changes in hypoxic ventilatory responses with advancing age in normal rats (Fukuda, 1992). Fukuda (1992) reported that ventilatory responses (normalized for body weight) to isocapnic hypoxia decline in parallel with advancing age in anaesthetized rats. The results of the present study suggest that the attenuated hypoxic ventilatory responses in geriatric rats (∼20 months old) reported by Fukuda (1992) might be caused by changes in pulmonary mechanics, metabolic rate or gas exchange, and not by attenuation of central respiratory drive. Alternatively, there might be rat strain-related differences in aging effects on the hypoxic ventilatory response since Fukuda used Wistar whereas we used Sprague-Dawley rats.
In addition to hypoxic ventilatory and phrenic responses, carotid chemoreceptor afferent responses to transient asphyxia and intravenous NaCN injection were also greatly attenuated in perinatal treated rats, suggesting impaired carotid chemotransduction (Ling et al. 1997c). On the other hand, phrenic responses to electrical stimulation of carotid sinus nerve were virtually unchanged in perinatal treated rats, suggesting that the central integration of carotid chemoafferent inputs is normal (Ling et al. 1997a). We argued that non-specific hyperoxic toxicity does not play a critical role in hyperoxia-induced functional impairment (Ling et al. 1996). Our current explanation is that perinatal hyperoxia was sufficient to suppress sensory activity from carotid chemoreceptors during a critical developmental period. Thus, the activity-dependent neuronal growth and/or synaptic enhancement necessary for proper elaboration in the chemical transduction of carotid bodies may be diminished. In support of this hypothesis, anatomical studies revealed a deficit in tyrosine hydroxylase expression in petrosal chemoafferent neurons, marked carotid body hypoplasia and a significant decrease in the number of axons in the carotid sinus nerve (Erickson et al. 1998). Although we cannot yet causally link these latter observations to the hyperoxia-induced functional impairment, these observations provide useful insights concerning the cellular and/or synaptic mechanisms that underlie this form of developmental plasticity. Functional recovery with advancing age may represent a (slow) regrowth of chemoafferent neurons innervating the carotid body, or a functional enhancement in central neural integration of the (still) reduced carotid chemoafferent inputs. However, the experimental design of the present study does not allow discrimination between these possibilities.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL 53319 and TG HL 07654).
References
- Antonini A, Stryker MP. Plasticity of geniculocortical afferents following brief or prolonged monocular occlusion in the cat. Journal of Comparative Neurology. 1996;369:64–82. doi: 10.1002/(SICI)1096-9861(19960520)369:1<64::AID-CNE5>3.0.CO;2-I. 10.1002/(SICI)1096-9861(19960520)369:1<64::AID-CNE5>3.3.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Gill-Kumar P, Millhorn DE. Input-output relationship of central neural circuits involved in respiration in cats. The Journal of Physiology. 1981;311:81–95. doi: 10.1113/jphysiol.1981.sp013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Mayer C, Jawa A, Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS, Katz DM. Chemoafferent degeneration and carotid body hypoplasia following chronic hyperoxia in newborn rats. The Journal of Physiology. 1998;509:519–526. doi: 10.1111/j.1469-7793.1998.519bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregosi R, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. The Journal of Physiology. 1994;477:469–479. doi: 10.1113/jphysiol.1994.sp020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y. Changes in ventilatory response to hypoxia in the rat during growth and aging. Pflügers Archiv. 1992;421:200–203. doi: 10.1007/BF00374827. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. The Journal of Physiology. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Jessell TM. Early experience and the fine tuning of synaptic connections. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 3. part X. New York: Elsevier; 1991. pp. 945–958. chap. 60. [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Attenuation of the hypoxic ventilatory response in adult rats following one month of perinatal hyperoxia. The Journal of Physiology. 1996;495:561–571. doi: 10.1113/jphysiol.1996.sp021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Integrated phrenic responses to carotid afferent stimulation in adult rats following perinatal hyperoxia. The Journal of Physiology. 1997a;500:787–796. doi: 10.1113/jphysiol.1997.sp022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Phrenic responses to isocapnic hypoxia in adult rats following perinatal hyperoxia. Respiration Physiology. 1997b;109:107–116. doi: 10.1016/s0034-5687(97)00045-5. [DOI] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Developmental plasticity of the hypoxic ventilatory response. Respiration Physiology. 1997c;110:261–268. doi: 10.1016/s0034-5687(97)00091-1. [DOI] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Perinatal hyperoxia suppresses hypoxic phrenic responses in adult rats: critical period and spontaneous recovery. Society for Neuroscience Abstracts. 1997d;23:435. [Google Scholar]
- Movshon JA. Reversal of the physiological effects of monocular deprivation in the kitten's visual cortex. The Journal of Physiology. 1976;261:125–174. doi: 10.1113/jphysiol.1976.sp011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]


