Figure 1. Structure of the TM6 region of CFTR.
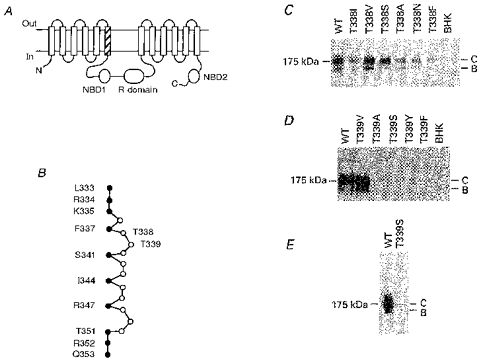
A, proposed overall topology of CFTR (as described by Riordan et al. 1989), comprising twelve membrane-spanning regions, two cytoplasmic nucleotide binding domains (NBDs) and the cytoplasmic regulatory (R) domain. The hatched area indicates TM6. N, N-terminus; C, C-terminus. B, primary sequence of TM6, after Cheung & Akabas (1996). Filled circles indicate those amino acids with side chains which have been proposed, on the basis of substituted cysteine accessibility mutagenesis, to line the aqueous lumen of the pore. According to this criterion, it has been proposed that neither T338 nor T339 have lumen-accessible side chains (Cheung & Akabas, 1996). C, immunoblots of T338 mutants stably expressed in BHK cells, carried out as described previously (Chang et al. 1993; Seibert et al. 1996). Equal amounts of total cellular protein were used in each lane. No CFTR protein was detected in untransfected cells (BHK, right-hand lane). Here and in D and E, the position of maltose binding protein-β-galactosidase from Escherichia coli (175 kDa marker) is shown on the left. Molecular mass markers below 100 kDa are not shown. WT, wild-type; B, core-glycosylated CFTR protein; C, fully glycosylated CFTR protein. D, immunoblots of T339 mutants stably expressed in BHK cells. Data shown are representative of at least ten experiments on different BHK cell colonies for each T339 mutant, over a total of four transfections for each mutant. E, Western blot of immunoprecipitated T339S protein, carried out as described in Methods. Similar results were observed with T339A, T339Y and T339F (not shown).
