Figure 2.
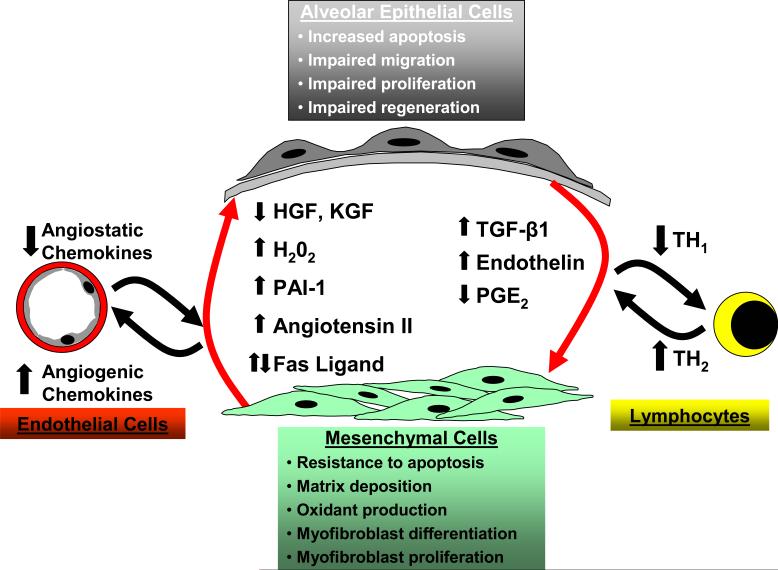
The pathogenesis of IPF is complex and three major hypotheses or paradigms for the pathogenesis of IPF have emerged: dysregulated epithelial-mesenchymal interactions, aberrant angiogenesis and the TH1/TH2 cytokine imbalance. Epithelial-mesenchymal interactions between altered epithelial and mesenchymal phenotypes results in dysregulated interactions between these cellular compartments. In response to an unknown stimulus, alveolar epithelial cells in IPF develop a phenotype characterized by increased apoptosis, dysregulated proliferation, impaired regeneration/differentiation and perhaps impaired migration. Alveolar epithelial cells elaborate increased TGF-β1 and endothelin-1 along with decreased levels of PGE2. Mesenchymal cells in this alveolar microenvironment acquire a contractile, synthetically active myofibroblast phenotype. Myofibroblasts retain the capacity to proliferate and are resistant to apoptosis while secreting large amounts of extracellular matrix proteins, soluble growth factors/cytokines and extracellular oxidants. Soluble mediators and the insoluble matrix elaborated by these myofibroblasts may, in turn, lead to aberrant reepithelialization that perpetuates a feed-forward cycle. IPF fibroblasts/myofibroblasts and alveolar epithelial cells contribute to an imbalance in angiogenic chemokines that may promote neovascularization and aberrant angiogenesis in IPF. A “shift” in the host immune response, favoring a TH2 cytokine profile, has been postulated to contribute to initiation and/or progression of IPF.